One-year safety and efficacy of intravenous etelcalcetide in patients on hemodialysis with secondary hyperparathyroidism
- PMID: 30859218
- PMCID: PMC7538239
- DOI: 10.1093/ndt/gfz039
One-year safety and efficacy of intravenous etelcalcetide in patients on hemodialysis with secondary hyperparathyroidism
Erratum in
-
Erratum.Nephrol Dial Transplant. 2020 Sep;35(9):1642. doi: 10.1093/ndt/gfz111. Epub 2019 May 15. Nephrol Dial Transplant. 2020. PMID: 31093680 Free PMC article. No abstract available.
Abstract
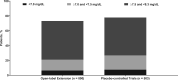
Background: Secondary hyperparathyroidism (sHPT), a common complication of chronic kidney disease, is characterized by elevated serum parathyroid hormone (PTH). Etelcalcetide is an intravenous calcimimetic that increases sensitivity of the calcium-sensing receptor to calcium and decreases PTH secretion. This open-label extension (OLE) trial evaluated the long-term effects of etelcalcetide for sHPT treatment in patients receiving hemodialysis.
Methods: This 52-week, multicenter, single-arm OLE enrolled patients from three parent trials: two randomized, double-blind, placebo-controlled trials and one open-label, single-arm, 'switch' study from cinacalcet to etelcalcetide. The primary endpoint was to investigate the nature, frequency, severity and relation to treatment of all adverse events (AEs) reported throughout the trial. Secondary endpoints included the proportion of patients with >30% reduction from baseline in PTH and the percentage change from baseline in PTH, albumin-corrected calcium (Ca), phosphate (P) and the calcium-phosphate product (Ca × P).ClinicalTrials.gov identifier: NCT01785875; Amgen study: 20120231.
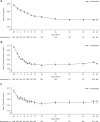
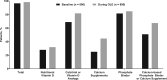
Results: Overall, 89.8% of the patients experienced one or more treatment-emergent AE. The most common were decreased blood Ca (43.3%), diarrhea (10.8%), vomiting (10.4%) and nausea (9.6%); symptomatic hypocalcemia occurred in 3.7% of the patients. Approximately 68% of patients achieved >30% reduction in PTH, and ∼56% achieved PTH ≤300 pg/mL. Mean percent changes from baseline ranged from -25.4% to -26.1% for PTH, -8.3% to -9.1% for Ca, -3.6% to -4.1% for P and -12.0% to -12.6% for Ca × P.
Conclusions: Etelcalcetide effectively lowered PTH and its effect was sustained, while no new safety concerns emerged over a 1-year treatment period.
Keywords: chronic kidney disease; etelcalcetide; open-label extension; safety, secondary hyperparathyroidism.
© The Author(s) 2019. Published by Oxford University Press on behalf of ERA-EDTA.
Figures


References
-
- Cunningham J, Locatelli F, Rodriguez M.. Secondary hyperparathyroidism: pathogenesis, disease progression, and therapeutic options. Clin J Am Soc Nephrol 2011; 6: 913–921 - PubMed
-
- Goodman WG, Quarles LD.. Development and progression of secondary hyperparathyroidism in chronic kidney disease: lessons from molecular genetics. Kidney Int 2008; 74: 276–288 - PubMed
-
- De Boer IH, Gorodetskaya I, Young B. et al. The severity of secondary hyperparathyroidism in chronic renal insufficiency is GFR-dependent, race-dependent, and associated with cardiovascular disease. J Am Soc Nephrol 2002; 13: 2762–2769 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

