Liver fibrosis imaging: A clinical review of ultrasound and magnetic resonance elastography
- PMID: 30859677
- PMCID: PMC6742585
- DOI: 10.1002/jmri.26716
Liver fibrosis imaging: A clinical review of ultrasound and magnetic resonance elastography
Abstract
Liver fibrosis is a histological hallmark of most chronic liver diseases, which can progress to cirrhosis and liver failure, and predisposes to hepatocellular carcinoma. Accurate diagnosis of liver fibrosis is necessary for prognosis, risk stratification, and treatment decision-making. Liver biopsy, the reference standard for assessing liver fibrosis, is invasive, costly, and impractical for surveillance and treatment response monitoring. Elastography offers a noninvasive, objective, and quantitative alternative to liver biopsy. This article discusses the need for noninvasive assessment of liver fibrosis and reviews the comparative advantages and limitations of ultrasound and magnetic resonance elastography techniques with respect to their basic concepts, acquisition, processing, and diagnostic performance. Variations in clinical contexts of use and common pitfalls associated with each technique are considered. In addition, current challenges and future directions to improve the diagnostic accuracy and clinical utility of elastography techniques are discussed. Level of Evidence: 5 Technical Efficacy Stage: 2 J. Magn. Reson. Imaging 2020;51:25-42.
© 2019 International Society for Magnetic Resonance in Medicine.
Conflict of interest statement
The authors report no conflicts of interest for this work.
Figures



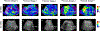
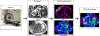
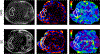
References
-
- Arthur MJP: Reversibility of liver fibrosis and cirrhosis following treatment for hepatitis C. Gastroenterology 2002; 122:1525–8. - PubMed
-
- Brunt EM, Janney CG, Bisceglie AM, et al.: Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol 1999; 94:2467–2474. - PubMed
-
- Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. The French METAVIR Cooperative Study Group. Hepatology 1994; 20(1 Pt 1):15–20. - PubMed
-
- Ratziu V, Charlotte F, Heurtier A, et al.: Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology 2005; 128:1898–906. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

