Dendritic shrinkage after injury: a cellular killer or a necessity for axonal regeneration?
- PMID: 30860164
- PMCID: PMC6524513
- DOI: 10.4103/1673-5374.253505
Dendritic shrinkage after injury: a cellular killer or a necessity for axonal regeneration?
Abstract
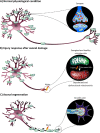
Dendrites form an essential component of the neuronal circuit have been largely overlooked in regenerative research. Nevertheless, subtle changes in the dendritic arbors of neurons are one of the first stages of various neurodegenerative diseases, leading to dysfunctional neuronal networks and ultimately cellular death. Maintaining dendrites is therefore considered an essential neuroprotective strategy. This mini-review aims to discuss an intriguing hypothesis, which postulates that dendritic shrinkage is an important stimulant to boost axonal regeneration, and thus that preserving dendrites might not be the ideal therapeutic method to regain a full functional network upon central nervous system damage. Indeed, our study in zebrafish, a versatile animal model with robust regenerative capacity recently unraveled that dendritic retraction is evoked prior to axonal regrowth after optic nerve injury. Strikingly, inhibiting dendritic pruning upon damage perturbed axonal regeneration. This constraining effect of dendrites on axonal regrowth has sporadically been proposed in literature, as summarized in this short narrative. In addition, the review discusses a plausible underlying mechanism for the observed antagonistic axon-dendrite interplay, which is based on energy restriction inside neurons. Axonal injury indeed leads to a high local energy demand in which efficient axonal energy supply is fundamental to ensure regrowth. At the same time, axonal lesion is known to induce mitochondrial depolarization, causing energy depletion in the axonal compartment of damaged neurons. Mitochondria, however, become mostly stationary after development, which has been proposed as a potential underlying reason for the low regenerative capacity of adult mammals. Per contra, upon reduced neuronal activity, mitochondrial mobility enhances. In this view, dendritic shrinkage after axonal injury in zebrafish could result in less synaptic input and hence, a release of mitochondria within the soma-dendrite compartment that then translocate to the axonal growth cone to stimulate axonal regeneration. If this hypothesis proofs to be correct, i.e. dendritic remodeling serving as fuel for axonal regeneration, we envision a major shift in the research focus within the neuroregenerative field and in the potential uncovering of various novel therapeutic targets.
Keywords: axonal regeneration; central nervous system; dendritic remodeling; energy supply; mitochondrial dynamics; mitochondrial transport; retina; zebrafish.
Conflict of interest statement
None
Figures

References
-
- Beckers A, Van Dyck A, Bollaerts I, Van Houcke J, Lefevere E, Andries L, Agostinone J, Van Hove I, Di Polo A, Lemmens K, Moons L. An antagonistic axon-dendrite interplay enables efficient neuronal repair in the adult Zebrafish central nervous system. Mol Neurobiol. 2018 doi: 10.1007/s12035-018-1292-5. - PubMed
-
- Cartoni R, Norsworthy MW, Bei F, Wang C, Li S, Zhang Y, Gabel CV, Schwarz TL, He Z. The mammalian-specific protein Armcx1 regulates mitochondrial transport during axon regeneration. Neuron. 2017;94:689. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

