CD19 CAR T cell product and disease attributes predict leukemia remission durability
- PMID: 30860496
- PMCID: PMC6486329
- DOI: 10.1172/JCI125423
CD19 CAR T cell product and disease attributes predict leukemia remission durability
Abstract
Background: Chimeric antigen receptor (CAR) T cells can induce remission in highly refractory leukemia and lymphoma subjects, yet the parameters for achieving sustained relapse-free survival are not fully delineated.
Methods: We analyzed 43 pediatric and young adult subjects participating in a Phase I trial of defined composition CD19CAR T cells (NCT02028455). CAR T cell phenotype, function and expansion, as well as starting material T cell repertoire, were analyzed in relation to therapeutic outcome (defined as achieving complete remission within 63 days) and duration of leukemia free survival and B cell aplasia.
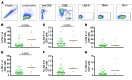
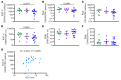
Results: These analyses reveal that initial therapeutic failures (n = 5) were associated with attenuated CAR T cell expansion and/or rapid attrition of functional CAR effector cells following adoptive transfer. The CAR T products were similar in phenotype and function when compared to products resulting in sustained remissions. However, the initial apheresed peripheral blood T cells could be distinguished by an increased frequency of LAG-3+/TNF-αlow CD8 T cells and, following adoptive transfer, the rapid expression of exhaustion markers. For the 38 subjects who achieved an initial sustained MRD-neg remission, remission durability correlated with therapeutic products having increased frequencies of TNF-α-secreting CAR CD8+ T cells, and was dependent on a sufficiently high CD19+ antigen load at time of infusion to trigger CAR T cell proliferation.
Conclusion: These parameters have the potential to prospectively identify patients at risk for therapeutic failure and support the development of approaches to boost CAR T cell activation and proliferation in patients with low levels of CD19 antigen.
Trial registration: ClinicalTrials.gov NCT02028455.
Funding: Partial funding for this study was provided by Stand Up to Cancer & St. Baldrick's Pediatric Dream Team Translational Research Grant (SU2C-AACR-DT1113), RO1 CA136551-05, Alex Lemonade Stand Phase I/II Infrastructure Grant, Conquer Cancer Foundation Career Development Award, Washington State Life Sciences Discovery Fund, Ben Towne Foundation, William Lawrence & Blanche Hughes Foundation, and Juno Therapeutics, Inc., a Celgene Company.
Keywords: Cancer immunotherapy; Immunology; Leukemias; Oncology.
Conflict of interest statement
Figures




Comment in
-
Improving CAR T cell immunotherapy-mediated remissions for pediatric leukemia.J Clin Invest. 2019 Apr 15;129(5):1842-1844. doi: 10.1172/JCI128743. eCollection 2019 Apr 15. J Clin Invest. 2019. PMID: 30985293 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

