Structure and Reactivity of Highly Twisted N-Acylimidazoles
- PMID: 30860852
- PMCID: PMC6461376
- DOI: 10.1021/acs.orglett.9b00624
Structure and Reactivity of Highly Twisted N-Acylimidazoles
Abstract
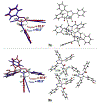
A modular and efficient synthesis of highly twisted N-acylimidazoles is reported. These twist amides were characterized via X-ray crystallography, NMR spectroscopy, IR spectroscopy, and DFT calculations. Modification of the substituent proximal to the amide revealed a maximum torsional angle of 88.6° in the solid state, which may be the most twisted amide reported for a nonbicyclic system to date. Reactivity and stability studies indicate that these twisted N-acylimidazoles may be valuable, namely as acyl transfer reagents.
Figures
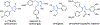
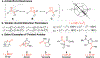

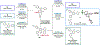
Similar articles
-
Acyclic Twisted Amides.Chem Rev. 2021 Oct 27;121(20):12746-12783. doi: 10.1021/acs.chemrev.1c00225. Epub 2021 Aug 18. Chem Rev. 2021. PMID: 34406005 Free PMC article. Review.
-
Biimidazole and bis(amide)bipyridine molybdenum carbonyl complexes as anions receptors.Inorg Chem. 2007 Apr 2;46(7):2846-53. doi: 10.1021/ic0621650. Epub 2007 Feb 16. Inorg Chem. 2007. PMID: 17302409
-
Structures of the Most Twisted Thioamide and Selenoamide: Effect of Higher Chalcogens of Twisted Amides on N-C(X) Resonance.Angew Chem Int Ed Engl. 2022 Aug 26;61(35):e202207346. doi: 10.1002/anie.202207346. Epub 2022 Jul 26. Angew Chem Int Ed Engl. 2022. PMID: 35776856 Free PMC article.
-
Reaction mechanism of the acidic hydrolysis of highly twisted amides: Rate acceleration caused by the twist of the amide bond.J Phys Chem B. 2006 Aug 3;110(30):15000-11. doi: 10.1021/jp0604037. J Phys Chem B. 2006. PMID: 16869615
-
Stability of medium-bridged twisted amides in aqueous solutions.J Org Chem. 2009 Mar 6;74(5):1869-75. doi: 10.1021/jo802192v. J Org Chem. 2009. PMID: 19178141 Free PMC article.
Cited by
-
Challenges and Opportunities in the Crusade of BRAF Inhibitors: From 2002 to 2022.ACS Omega. 2023 Jul 26;8(31):27819-27844. doi: 10.1021/acsomega.3c00332. eCollection 2023 Aug 8. ACS Omega. 2023. PMID: 37576670 Free PMC article. Review.
-
Acyclic Twisted Amides.Chem Rev. 2021 Oct 27;121(20):12746-12783. doi: 10.1021/acs.chemrev.1c00225. Epub 2021 Aug 18. Chem Rev. 2021. PMID: 34406005 Free PMC article. Review.
References
-
- Olson GL; Bolin DR; Bonner MP; Bos M; Cook CM; Fry DC; Graves BJ; Hatada M; Hill DE Concepts and progress in the development of peptide mimetics. J. Med. Chem 1993, 36, 3039–3049; - PubMed
- Moore GJ Designing peptide mimetics. Trends Pharmacol. Sci 1994, 15, 124–129; - PubMed
- Beltrán M; Pedroso E; Grandas A A comparison of histidine protecting groups in the synthesis of peptide-oligonucleotide conjugates. Tetrahedron Lett 1998, 39, 4115–4118;
- Lidia De L Naturally Occurring and Synthetic Imidazoles: Their Chemistry and Their Biological Activities. Curr. Med. Chem 2006, 13, 1–23; - PubMed
- Gupta RR; Kumar M; Gupta V, Heterocyclic Chemistry Springer: 1999; Vol. II;
- Bellina F; Cauteruccio S; Rossi R Synthesis and biological activity of vicinal diaryl-substituted 1H-imidazoles. Tetrahedron 2007, 63, 4571–4624;
- Jin Z Muscarine, imidazole, oxazole and thiazole alkaloids. Nat. Prod. Rep 2009, 26, 382–445; - PubMed
- Girish KG; Vinod K; Kamalneet K Imidazole Containing Natural Products as Antimicrobial Agents: A Review. J. Nat. Prod 2014, 4, 73–81.
-
- Takle AK; Bamford MJ; Davies S; Davis RP; Dean DK; Gaiba A; Irving EA; King FD; Naylor A; Parr CA; Ray AM; Reith AD; Smith BB; Staton PC; Steadman JGA; Stean TO; Wilson DM The identification of potent, selective and CNSpenetrant furan-based inhibitors of B-Raf kinase. Bioorganic Med. Chem. Lett 2008, 18, 4373–4376. - PubMed
-
- Copeland GT; Jarvo ER; Miller SJ Minimal Acylase-Like Peptides. Conformational Control of Absolute Stereospecificity. J. Org. Chem 1998, 63, 6784–6785; - PubMed
- Jarvo ER; Copeland GT; Papaioannou N; Bonitatebus PJ; Miller SJ A Biomimetic Approach to Asymmetric Acyl Transfer Catalysis. J. Am. Chem. Soc 1999, 121, 11638–11643;
- Sculimbrene BR; Morgan AJ; Miller SJ Nonenzymatic peptide-based catalytic asymmetric phosphorylation of inositol derivatives. Chem. Commun 2003, 1781–1785; - PubMed
- Lewis CA; Sculimbrene BR; Xu Y; Miller SJ Desymmetrization of Glycerol Derivatives with Peptide-Based Acylation Catalysts. Org. Lett 2005, 7, 3021–3023; - PubMed
- Heller ST; Sarpong R Chemoselective Esterification and Amidation of Carboxylic Acids with Imidazole Carbamates and Ureas. Org. Lett 2010, 12, 4572–4575; - PubMed
- Heller ST; Schultz EE; Sarpong R Chemoselective N-Acylation of Indoles and Oxazolidinones with Carbonylazoles. Angew. Chem. Int. Ed 2012, 51, 8304–8308; - PubMed
- Lu Y; Wei P; Pei Y; Xu H; Xin X; Pei Z Regioselective acetylation of carbohydrates and diols catalyzed by tetramethyl-ammonium hydroxide in water. Green Chem 2014, 16, 4510–4514.
-
- Long TE Designing Imidazole-Based Ionic Liquids and Ionic Liquid Monomers for Emerging Technologies AU - Green, Matthew D. Polym. Rev 2009, 49, 291–314;
- Troshenkova SV; Sashina ES; Novoselov NP; Arndt K-F; Jankowsky S Structure of ionic liquids on the basis of imidazole and their mixtures with water. Russ. J. Gen. Chem 2010, 80, 106–111;
- Tao Y; Dong R; Pavlidis IV; Chen B; Tan T Using imidazolium-based ionic liquids as dual solvent-catalysts for sustainable synthesis of vitamin esters: inspiration from bio- and organo-catalysis. Green Chem 2016, 18, 1240–1248;
- Mu X; Jiang N; Liu C; Zhang D New Insight into the Formation Mechanism of Imidazolium-Based Ionic Liquids from N-Alkyl Imidazoles and Halogenated Hydrocarbons: A Polar Microenvironment Induced and Autopromoted Process. J. Phys. Chem. A 2017, 121, 1133–1139; - PubMed
- Dreier TA; Ringstrand BS; Seifert S; Firestone MA Synthesis and application of a metal ion coordinating ionic liquid monomer: Towards size and dispersity control of nanoparticles formed within a structured polyelectrolyte. Eur. Polym. J 2018, 107, 275–286.
-
- Connors KA; Pandit NK N-Methylimidazole as a catalyst for analytical acetylations of hydroxy compounds. Anal. Chem 1978, 50, 1542–1545;
- Hojabri L; Hartikka A; Moghaddam FM; Arvidsson PI A New Imidazole-Containing Imidazolidinone Catalyst for Organocatalyzed Asymmetric Conjugate Addition of Nitroalkanes to Aldehydes. Adv. Synth. Catal 2007, 349, 740–748;
- Zhang Z; Xie F; Jia J; Zhang W Chiral Bicycle Imidazole Nucleophilic Catalysts: Rational Design, Facile Synthesis, and Successful Application in Asymmetric Steglich Rearrangement. J. Am. Chem. Soc 2010, 132, 15939–15941; - PubMed
- Li Y; Giulionatti M; Houghten RA Macrolactonization of Peptide Thioesters Catalyzed by Imidazole and Its Application in the Synthesis of Kahalalide B and Analogues. Org. Lett 2010, 12, 2250–2253; - PMC - PubMed
- Khan MN; Pal S; Karamthulla S; Choudhury LH Imidazole as organocatalyst for multicomponent reactions: diversity oriented synthesis of functionalized hetero- and carbocycles using in situ-generated benzylidenemalononitrile derivatives. RSC Adv 2014, 4, 3732–3741.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

