Spike burst-pause dynamics of Purkinje cells regulate sensorimotor adaptation
- PMID: 30860991
- PMCID: PMC6430425
- DOI: 10.1371/journal.pcbi.1006298
Spike burst-pause dynamics of Purkinje cells regulate sensorimotor adaptation
Abstract
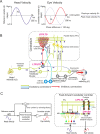
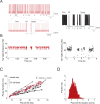
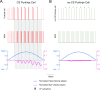
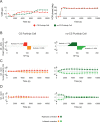
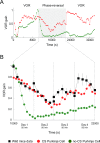
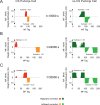
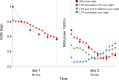
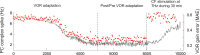
Cerebellar Purkinje cells mediate accurate eye movement coordination. However, it remains unclear how oculomotor adaptation depends on the interplay between the characteristic Purkinje cell response patterns, namely tonic, bursting, and spike pauses. Here, a spiking cerebellar model assesses the role of Purkinje cell firing patterns in vestibular ocular reflex (VOR) adaptation. The model captures the cerebellar microcircuit properties and it incorporates spike-based synaptic plasticity at multiple cerebellar sites. A detailed Purkinje cell model reproduces the three spike-firing patterns that are shown to regulate the cerebellar output. Our results suggest that pauses following Purkinje complex spikes (bursts) encode transient disinhibition of target medial vestibular nuclei, critically gating the vestibular signals conveyed by mossy fibres. This gating mechanism accounts for early and coarse VOR acquisition, prior to the late reflex consolidation. In addition, properly timed and sized Purkinje cell bursts allow the ratio between long-term depression and potentiation (LTD/LTP) to be finely shaped at mossy fibre-medial vestibular nuclei synapses, which optimises VOR consolidation. Tonic Purkinje cell firing maintains the consolidated VOR through time. Importantly, pauses are crucial to facilitate VOR phase-reversal learning, by reshaping previously learnt synaptic weight distributions. Altogether, these results predict that Purkinje spike burst-pause dynamics are instrumental to VOR learning and reversal adaptation.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Ito M. Cerebellar control of the VOR; around the flocculus hypothesis. Annu Rev Neurosci. 1982;5(1):275–97. - PubMed
-
- Lorente de Nó R. Vestibulo-ocular reflex arc. Archiv Neurol & Psychiatry. 1933.
-
- Cohen B. The VOR Arc In: Kornhuber HH, editor. Vestibular System Part 1: Basic Mechanisms: Springer; Berlin Heidelberg; 1974. p. 477–540.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

