Fosfomycin for Injection (ZTI-01) Versus Piperacillin-tazobactam for the Treatment of Complicated Urinary Tract Infection Including Acute Pyelonephritis: ZEUS, A Phase 2/3 Randomized Trial
- PMID: 30861061
- PMCID: PMC6880332
- DOI: 10.1093/cid/ciz181
Fosfomycin for Injection (ZTI-01) Versus Piperacillin-tazobactam for the Treatment of Complicated Urinary Tract Infection Including Acute Pyelonephritis: ZEUS, A Phase 2/3 Randomized Trial
Abstract
Background: ZTI-01 (fosfomycin for injection) is an epoxide antibiotic with a differentiated mechanism of action (MOA) inhibiting an early step in bacterial cell wall synthesis. ZTI-01 has broad in vitro spectrum of activity, including multidrug-resistant Gram-negative pathogens, and is being developed for treatment of complicated urinary tract infection (cUTI) and acute pyelonephritis (AP) in the United States.
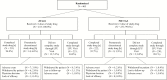
Methods: Hospitalized adults with suspected or microbiologically confirmed cUTI/AP were randomized 1:1 to 6 g ZTI-01 q8h or 4.5 g intravenous (IV) piperacillin-tazobactam (PIP-TAZ) q8h for a fixed 7-day course (no oral switch); patients with concomitant bacteremia could receive up to 14 days.
Results: Of 465 randomized patients, 233 and 231 were treated with ZTI-01 and PIP-TAZ, respectively. In the microbiologic modified intent-to-treat (m-MITT) population, ZTI-01 met the primary objective of noninferiority compared with PIP-TAZ with overall success rates of 64.7% (119/184 patients) vs 54.5% (97/178 patients), respectively; treatment difference was 10.2% (95% confidence interval [CI]: -0.4, 20.8). Clinical cure rates at test of cure (TOC, day 19-21) were high and similar between treatments (90.8% [167/184] vs 91.6% [163/178], respectively). In post hoc analysis using unique pathogens typed by pulsed-field gel electrophoresis, overall success rates at TOC in m-MITT were 69.0% (127/184) for ZTI-01 versus 57.3% (102/178) for PIP-TAZ (difference 11.7% 95% CI: 1.3, 22.1). ZTI-01 was well tolerated. Most treatment-emergent adverse events, including hypokalemia and elevated serum aminotransferases, were mild and transient.
Conclusions: ZTI-01 was effective for treatment of cUTI including AP and offers a new IV therapeutic option with a differentiated MOA for patients with serious Gram-negative infections.
Clinical trial registration: NCT02753946.
Keywords: ZTI-01; acute pyelonephritis; complicated urinary tract infection; fosfomycin.
© The Author(s) 2019. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures

Comment in
-
By ZEUS! Can We Use Intravenous Fosfomycin for Complicated Urinary Tract Infections?Clin Infect Dis. 2019 Nov 27;69(12):2057-2058. doi: 10.1093/cid/ciz185. Clin Infect Dis. 2019. PMID: 30839061 No abstract available.
References
-
- Alemayehu D, Quinn J, Cook J, Kunkel M, Knirsch CA. A paradigm shift in drug development for treatment of rare multidrug-resistant gram-negative pathogens. Clin Infect Dis 2012; 55:562–7. - PubMed
-
- Falagas ME, Maraki S, Karageorgopoulos DE, Kastoris AC, Mavromanolakis E, Samonis G. Antimicrobial susceptibility of multidrug-resistant (MDR) and extensively drug-resistant (XDR) Enterobacteriaceae isolates to fosfomycin. Int J Antimicrob Agents 2010; 35:240–3. - PubMed
-
- Monurol (fosfomycin tromethamine) sachet prescribing information, 2011 February. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/050717s005lbl.pdf. Accessed 21 September 2017.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

