Sinus venosus incorporation: contentious issues and operational criteria for developmental and evolutionary studies
- PMID: 30861129
- PMCID: PMC6481585
- DOI: 10.1111/joa.12962
Sinus venosus incorporation: contentious issues and operational criteria for developmental and evolutionary studies
Abstract
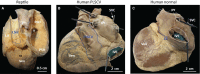
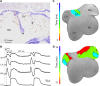
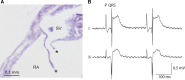
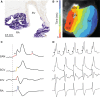
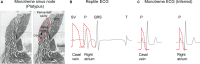
The sinus venosus is a cardiac chamber upstream of the right atrium that harbours the dominant cardiac pacemaker. During human heart development, the sinus venosus becomes incorporated into the right atrium. However, from the literature it is not possible to deduce the characteristics and importance of this process of incorporation, due to inconsistent terminology and definitions in the description of multiple lines of evidence. We reviewed the literature regarding the incorporation of the sinus venosus and included novel electrophysiological data. Most mammals that have an incorporated sinus venosus show a loss of a functional valve guard of the superior caval vein together with a loss of the electrical sinuatrial delay between the sinus venosus and the right atrium. However, these processes are not necessarily intertwined and in a few species only the sinuatrial delay may be lost. Sinus venosus incorporation can be characterised as the loss of the sinuatrial delay of which the anatomical and molecular underpinnings are not yet understood.
Keywords: development; evolution; heart; sinuatrial valve.
© 2019 The Authors. Journal of Anatomy published by John Wiley &; Sons Ltd on behalf of Anatomical Society.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures

Similar articles
-
Early morphogenesis of the sinuatrial region of the chick heart: a contribution to the understanding of the pathogenesis of direct pulmonary venous connections to the right atrium and atrial septal defects in hearts with right isomerism of the atrial appendages.Anat Rec (Hoboken). 2007 Feb;290(2):168-80. doi: 10.1002/ar.20418. Anat Rec (Hoboken). 2007. PMID: 17441209
-
Molecular pathway for the localized formation of the sinoatrial node.Circ Res. 2007 Feb 16;100(3):354-62. doi: 10.1161/01.RES.0000258019.74591.b3. Epub 2007 Jan 18. Circ Res. 2007. PMID: 17234970
-
Morpho-functional characterization of the systemic venous pole of the reptile heart.Sci Rep. 2017 Jul 27;7(1):6644. doi: 10.1038/s41598-017-06291-z. Sci Rep. 2017. PMID: 28751678 Free PMC article.
-
Development and structures of the venous pole of the heart.Dev Dyn. 2006 Jan;235(1):2-9. doi: 10.1002/dvdy.20578. Dev Dyn. 2006. PMID: 16193508 Review.
-
Current concepts of anatomy and electrophysiology of the sinus node.J Interv Card Electrophysiol. 2016 Jun;46(1):9-18. doi: 10.1007/s10840-016-0137-2. Epub 2016 May 3. J Interv Card Electrophysiol. 2016. PMID: 27142063 Review.
Cited by
-
Single-cell transcriptome analysis reveals CD34 as a marker of human sinoatrial node pacemaker cardiomyocytes.Nat Commun. 2024 Nov 27;15(1):10206. doi: 10.1038/s41467-024-54337-4. Nat Commun. 2024. PMID: 39604360 Free PMC article.
-
Intrahepatic and anterior course of the inferior vena cava: CT image and 3D reconstruction of a rare anatomical variation.Surg Radiol Anat. 2024 Mar;46(3):377-379. doi: 10.1007/s00276-023-03289-3. Epub 2024 Jan 27. Surg Radiol Anat. 2024. PMID: 38280967
-
Cardiac CT and Transesophageal Echocardiogram Evaluation of a Sinus Venosus-Type Atrial Septal Defect With Partial Anomalous Pulmonary Venous Return and a Persistent Left Superior Vena Cava.Cureus. 2021 Dec 12;13(12):e20367. doi: 10.7759/cureus.20367. eCollection 2021 Dec. Cureus. 2021. PMID: 35036201 Free PMC article.
-
High cardiomyocyte diversity in human early prenatal heart development.iScience. 2022 Dec 21;26(1):105857. doi: 10.1016/j.isci.2022.105857. eCollection 2023 Jan 20. iScience. 2022. PMID: 36624836 Free PMC article.
-
Opportunities and short-comings of the axolotl salamander heart as a model system of human single ventricle and excessive trabeculation.Sci Rep. 2022 Nov 28;12(1):20491. doi: 10.1038/s41598-022-24442-9. Sci Rep. 2022. PMID: 36443330 Free PMC article.
References
-
- Adams WE (1937) A contribution to the anatomy of the avian heart as seen in the Kiwi (Apteryx australis) and the Yellow‐creseted Penguin (Megadyptes antipodum). Proc Zool Soc London 107, 417–441.
-
- Benninghoff A (1933) Das Herz In: Handbuch Der Vergleichende Anatomie Der Wirbeltiere 6. (eds Bolk L, Göppert E, Kallius E, Lubosch W.), pp. 467–556. Berlin: Urban & Schwarzenberg.
-
- Bharucha T, Spicer DE, Mohun TJ, et al. (2015) Cor triatriatum or divided atriums: which approach provides the better understanding? Cardiol Young 25, 193–207. - PubMed
-
- Blom NA, Gittenberger‐de Groot AC, Jongeneel TH, et al. (2001) Normal development of the pulmonary veins in human embryos and formulation of a morphogenetic concept for sinus venosus defects. Am J Cardiol 87, 305–309. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

