Absence of axonal sprouting following unilateral lesion in 125-day-old rat supraoptic nucleus may be due to age-dependent decrease in protein levels of ciliary neurotrophic factor receptor alpha
- PMID: 30861131
- PMCID: PMC6656591
- DOI: 10.1002/cne.24675
Absence of axonal sprouting following unilateral lesion in 125-day-old rat supraoptic nucleus may be due to age-dependent decrease in protein levels of ciliary neurotrophic factor receptor alpha
Abstract
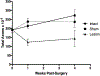
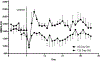
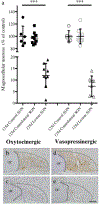
Within the supraoptic nucleus (SON) of a 35-day-old rat, we previously demonstrated a collateral sprouting response that reinnervates the partially denervated neural lobe (NL) after unilateral lesion of the hypothalamo-neurohypophysial tract. Others have shown a decreased propensity for axonal sprouting in an aged brain; therefore, to see if the SON exhibits a decreased propensity for axonal sprouting as the animal ages, we performed a unilateral lesion in the 125-day-old rat SON. Ultrastructural analysis of axon profiles in the NL of the 125-day-old rat demonstrated an absence of axonal sprouting following injury. We previously demonstrated that ciliary neurotrophic factor (CNTF) promotes process outgrowth from injured magnocellular neuron axons in vitro. Thus, we hypothesized that the lack of axonal sprouting in the 125-day-old rat SON may be due to a reduction in CNTF or the CNTF receptor components. To this point, we found that as the rat ages there is significantly less CNTF receptor alpha (CNTFRα) protein in the uninjured, 125-day-old rat compared to the uninjured, 35-day-old rat. We also observed that protein levels of CNTF and the CNTF receptor components were increased in the SON and NL following injury in the 35-day-old rat, but there was no difference in the protein levels in the 125-day-old rat. Altogether, the results presented herein demonstrate that the plasticity within the SON is highly dependent on the age of the rat, and that a decrease in CNTFRα protein levels in the 125-day-old rat may contribute to the loss of axonal sprouting following axotomy.
Keywords: CNTF; CNTFRα; RRID:AB_2136105; RRID:AB_2276485; RRID:AB_397120; RRID:AB_476697; RRID:AB_518526; RRID:AB_518680; RRID:AB_631590; aging; axonal sprouting; neural lobe; supraoptic nucleus.
© 2019 Wiley Periodicals, Inc.
Figures
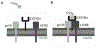



References
-
- Armstrong WE, & Hatton GI (1978). Morphological changes in the rat supraoptic and paraventricular nuclei during the diurnal cycle. Brain Research, 157(2), 407. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

