The Role of Hypoxia in Corneal Extracellular Matrix Deposition and Cell Motility
- PMID: 30861330
- PMCID: PMC8827063
- DOI: 10.1002/ar.24110
The Role of Hypoxia in Corneal Extracellular Matrix Deposition and Cell Motility
Abstract
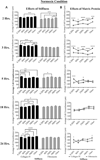
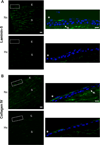
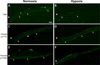
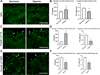
The cornea is an excellent model tissue to study how cells adapt to periods of hypoxia as it is naturally exposed to diurnal fluxes in oxygen. It is avascular, transparent, and highly innervated. In certain pathologies, such as diabetes, limbal stem cell deficiency, or trauma, the cornea may be exposed to hypoxia for variable lengths of time. Due to its avascularity, the cornea requires atmospheric oxygen, and a reduction in oxygen availability can impair its physiology and function. We hypothesize that hypoxia alters membrane stiffness and the deposition of matrix proteins, leading to changes in cell migration, focal adhesion formation, and wound repair. Two systems-a 3D corneal organ culture model and polyacrylamide substrates of varying stiffness-were used to examine the response of corneal epithelium to normoxic and hypoxic environments. Exposure to hypoxia alters the deposition of the matrix proteins such as laminin and Type IV collagen. In addition, previous studies had shown a change in fibronectin after injury. Studies performed on matrix-coated acrylamide substrates ranging from 0.2 to 50 kPa revealed stiffness-dependent changes in cell morphology. The localization, number, and length of paxillin pY118- and vinculin pY1065-containing focal adhesions were different in wounded corneas and in human corneal epithelial cells incubated in hypoxic environments. Overall, these results demonstrate that low-oxygenated environments modify the composition of the extracellular matrix, basal lamina stiffness, and focal adhesion dynamics, leading to alterations in the function of the cornea. Anat Rec, 2019. © 2019 Wiley Periodicals, Inc.
Keywords: cornea; epithelium; extracellular matrix; focal adhesions; hypoxia.
© 2019 Wiley Periodicals, Inc.
Figures




Similar articles
-
Hypoxia modulates the development of a corneal stromal matrix model.Exp Eye Res. 2018 May;170:127-137. doi: 10.1016/j.exer.2018.02.021. Epub 2018 Feb 27. Exp Eye Res. 2018. PMID: 29496505 Free PMC article.
-
Epithelial cells exert differential traction stress in response to substrate stiffness.Exp Eye Res. 2019 Apr;181:25-37. doi: 10.1016/j.exer.2019.01.014. Epub 2019 Jan 14. Exp Eye Res. 2019. PMID: 30653966 Free PMC article.
-
Quantitative analysis of the effects of extracellular matrix proteins on membrane dynamics associated with corneal epithelial cell motility.Invest Ophthalmol Vis Sci. 2010 Sep;51(9):4492-9. doi: 10.1167/iovs.09-4380. Epub 2010 Mar 31. Invest Ophthalmol Vis Sci. 2010. PMID: 20357207
-
Targeting Ca2+-dependent pathways to promote corneal epithelial wound healing induced by CISD2 deficiency.Cell Signal. 2023 Sep;109:110755. doi: 10.1016/j.cellsig.2023.110755. Epub 2023 Jun 12. Cell Signal. 2023. PMID: 37315750 Review.
-
[The cornea: stasis and dynamics].Nippon Ganka Gakkai Zasshi. 2008 Mar;112(3):179-212; discussion 213. Nippon Ganka Gakkai Zasshi. 2008. PMID: 18411711 Review. Japanese.
Cited by
-
Hypoxia Induced Heparan Sulfate Primes the Extracellular Matrix for Endothelial Cell Recruitment by Facilitating VEGF-Fibronectin Interactions.Int J Mol Sci. 2019 Oct 12;20(20):5065. doi: 10.3390/ijms20205065. Int J Mol Sci. 2019. PMID: 31614727 Free PMC article.
-
The donation-transplantation process and corneal graft failure: A case-control study.PLoS One. 2025 May 22;20(5):e0321225. doi: 10.1371/journal.pone.0321225. eCollection 2025. PLoS One. 2025. PMID: 40402957 Free PMC article.
-
Oxygenated Exosome-Based Nanoeyedrop for Mitigating Hypoxia in Corneal Wound Healing: Impact on Healing Properties of Human Corneal Epithelial Cells.ACS Pharmacol Transl Sci. 2025 Jan 21;8(2):602-612. doi: 10.1021/acsptsci.4c00724. eCollection 2025 Feb 14. ACS Pharmacol Transl Sci. 2025. PMID: 39974628
-
Hypoxia adaptation in the cornea: Current animal models and underlying mechanisms.Animal Model Exp Med. 2021 Nov 28;4(4):300-310. doi: 10.1002/ame2.12192. eCollection 2021 Dec. Animal Model Exp Med. 2021. PMID: 34977481 Free PMC article. Review.
-
Changes in Epithelial and Stromal Corneal Stiffness Occur with Age and Obesity.Bioengineering (Basel). 2020 Feb 7;7(1):14. doi: 10.3390/bioengineering7010014. Bioengineering (Basel). 2020. PMID: 32046198 Free PMC article.
References
-
- Alenghat FJ, Fabry B, Tsai KY, Goldmann WH, Ingber DE. 2000. Analysis of cell mechanics in single vinculin-deficient cells using a magnetic tweezer. Biochem Biophys Res Commun 277:93–99. - PubMed
-
- Azar DT, Spurr-Michaud SJ, Tisdale AS, Gipson IK. 1992. Altered epithelial-basement membrane interactions in diabetic corneas. Arch Ophthalmol 110:537–540. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

