Role of Cell Surface Lipids and Thiol-Disulphide Exchange Pathways in Regulating the Encryption and Decryption of Tissue Factor
- PMID: 30861549
- PMCID: PMC7805104
- DOI: 10.1055/s-0039-1681102
Role of Cell Surface Lipids and Thiol-Disulphide Exchange Pathways in Regulating the Encryption and Decryption of Tissue Factor
Abstract
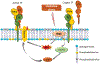
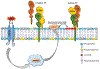
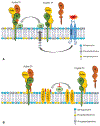
Tissue factor (TF), a transmembrane glycoprotein, is the cellular receptor of the coagulation factors VII (FVII) and VIIa (FVIIa). The formation of TF-FVIIa complex triggers the initiation of the blood coagulation pathway. TF plays an essential role in haemostasis, but an aberrant expression of TF activity contributes to thrombotic disorders. In health, TF pro-coagulant activity on cells is controlled tightly to allow sufficient coagulant activity to achieve haemostasis but not to cause thrombosis. It is achieved largely by selective localization of TF in the body and encryption of TF at the cell surface. A vast majority of TF on resting cells exists in an encrypted state with minimal pro-coagulant activity but becomes pro-thrombotic following cell injury or activation. At present, the mechanisms that are responsible for TF encryption and activation (decryption) are not entirely clear, but recent studies provide important mechanistic insights into these processes. To date, externalization of phosphatidylserine to the outer leaflet and thiol-disulphide exchange pathways that either turn on and off the allosteric disulphide bond in TF are shown to play a major role in regulating TF pro-coagulant activity on cell surfaces. Recent studies showed that sphingomyelin, a major phospholipid in the outer leaflet of plasma membrane, plays a critical role in the encryption of TF in resting cells. The present review provides an overview of recent literature on the above-described mechanisms of TF encryption and decryption with a particular emphasis on our recent findings.
Georg Thieme Verlag KG Stuttgart · New York.
Conflict of interest statement
None declared.
Figures
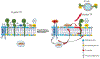
References
-
- Rapaport SI, Rao LVM. The tissue factor pathway: how it has become a “prima ballerina”. Thromb Haemost 1995;74(01): 7–17 - PubMed
-
- Fleck RA, Rao LVM, Rapaport SI, Varki N. Localization of human tissue factor antigen by immunostaining with monospecific, polyclonal anti-human tissue factor antibody. Thromb Res 1990;59(02):421–437 - PubMed
-
- Butenas S, Bouchard BA, Brummel-Ziedins KE, Parhami-Seren B, Mann KG. Tissue factor activity in whole blood. Blood 2005;105 (07):2764–2770 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

