Comparison of indirect peroxidase and avidin-biotin-peroxidase complex (ABC) immunohistochemical staining procedures for c-fos in rat brain
- PMID: 30861576
- PMCID: PMC6539692
- DOI: 10.1111/joa.12967
Comparison of indirect peroxidase and avidin-biotin-peroxidase complex (ABC) immunohistochemical staining procedures for c-fos in rat brain
Abstract
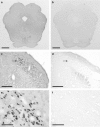
c-Fos is the product of a gene expressed within neurons in the brain that serves as an anatomical marker of cellular activation. Immunohistochemical staining for c-fos allows a characterization of the effects of many different types of experimental manipulations on neuronal activity, making it a powerful technique for understanding brain, drug and behavior relationships. This study compared visualization of an anti-c-fos primary antibody in 40-μm-thick cryostat sections of formaldehyde-fixed rat brainstem using either a peroxidase enzyme-conjugated secondary antibody (indirect peroxidase) or the peroxidase-conjugated avidin-biotin complex (ABC) method. All sections were treated with H2 O2 to quench endogenous peroxidase enzyme and sodium borohydride to enhance permeability of the tissue and improve staining quality. Every other section was used to examine either the indirect peroxidase or the ABC method. Sections for the indirect peroxidase method were treated with Triton X-100 detergent to increase tissue permeability, goat serum to reduce non-specific binding of the secondary antibody and, in some cases, bovine serum albumin (BSA) to reduce non-specific binding of the primary antibody. Sections for the ABC method were treated with dilute normal serum, and avidin and biotin solutions and, in some cases BSA. Alternate sections were incubated for 72 h in either rabbit anti-c-fos primary antibody (1 : 20 000) or its vehicle (negative control). For the indirect peroxidase protocol, tissues were treated with peroxidase-conjugated goat anti-rabbit secondary antibody. For the ABC protocol, tissues were treated with biotinylated goat anti-rabbit secondary antibody and ABC peroxidase complex. All sections were reacted with 3,3'-diaminobenzadine (DAB) and H2 O2 , mounted and coverslipped. Both methods produced specific staining of c-fos-containing neurons, relative to the negative control sections. The indirect peroxidase protocol produced clear staining of c-fos-containing neurons, with very little background in the negative control sections. Staining for c-fos was enhanced using the ABC method in that c-fos stained neurons were darker and more clearly visible after shorter treatment with DAB. However, negative control sections showed a greater amount of non-specific staining with the ABC method. Thus, the ABC method was more sensitive but showed reduced specificity, with BSA treatment slightly reducing the level of non-specific staining. Overall, the ABC method produced better visualization and contrast of c-fos-containing neurons against the background color of the tissue.
Keywords: avidin-biotin-peroxidase complex; c-fos; immunohistochemistry.
© 2019 Anatomical Society.
Figures


References
-
- Curran T, Morgan JI (1995) Fos: an immediate‐early transcription factor in neurons. J Neurobiol 26, 403–412. - PubMed
-
- Davoodi N, te Riele P, Langlois X (2014) Examining dopamine D3 receptor occupancy by antipsychotic drugs via [3H]7‐OH‐DPAT ex vivo autoradiography and its cross‐validation via c‐fos immunohistochemistry in the rat brain. Eur J Pharmacol 740, 669–675. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

