Expression of ventral telencephalon transcription factors ASCL1 and DLX2 in the early fetal human cerebral cortex
- PMID: 30861584
- PMCID: PMC6704271
- DOI: 10.1111/joa.12971
Expression of ventral telencephalon transcription factors ASCL1 and DLX2 in the early fetal human cerebral cortex
Abstract
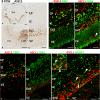
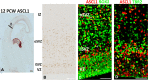
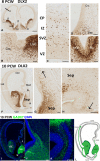
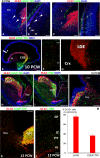
In rodent ventral telencephalon, diffusible morphogens induce expression of the proneural transcription factor ASCL1, which in turn induces expression of the transcription factor DLX2 that controls differentiation of cortical interneuron precursors and their tangential migration to the cerebral cortex. RNAseq analysis of human fetal samples of dorsal telencephalon revealed consistently high cortical expression of ASCL1 and increasing expression of DLX2 between 7.5 and 17 post-conceptional weeks (PCW). We explored whether cortical expression of these genes represented a population of intracortically derived interneuron precursors. Immunohistochemistry revealed an ASCL1+ /DLX2+ population of progenitor cells in the human ganglionic eminences between 6.5 and 12 PCW, but in the cortex there also existed a population of ASCL1+ /DLX2- progenitors in the subventricular zone (SVZ) that largely co-expressed cortical markers PAX6 or TBR2, although a few ASCL1+ /PAX6- progenitors were observed in the ventricular zone (VZ) and ASCL1+ cells expressing the interneuron marker GAD67 were present in the SVZ. Although rare in the VZ, DLX2+ cells progressively increased in number between 8 and 12 PCW across the cortical wall and the majority co-expressed LHX6 and originated either in the MGE, migrating to the lateral cortex, or from the septum, populating the medial wall. A minority co-expressed COUP-TFII, which identifies cells from the caudal ganglionic eminence (CGE). By 19 PCW, a significant increase in expression of DLX2 and ASCL1 was observed in the cortical VZ with a small proportion expressing both proteins. The DLX2+ cells did not co-express a cell division marker, so were not progenitors. The majority of DLX2+ cells throughout the cortical plate expressed COUP-TFII rather than LHX6+ . As the VZ declined as a proliferative zone it appeared to be re-defined as a migration pathway for COUP-TFII+ /DLX2+ interneurons from CGE to cortex. Therefore, in developing human cortex, ASCL1 expression predominantly marks a population of intermediate progenitors giving rise to glutamatergic neurons. DLX2 expression predominantly defines post-mitotic interneuron precursors.
Keywords: cortical inhibitory interneurons; development; dorsal telencephalon; ganglionic eminences.
© 2019 The Authors. Journal of Anatomy published by John Wiley & Sons Ltd on behalf of Anatomical Society.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures



References
-
- Anderson SA, Eisenstat DD, Shi L, et al. (1997) Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science 278, 474–476. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

