Dietary Eriodictyol Alleviates Adiposity, Hepatic Steatosis, Insulin Resistance, and Inflammation in Diet-Induced Obese Mice
- PMID: 30862092
- PMCID: PMC6429409
- DOI: 10.3390/ijms20051227
Dietary Eriodictyol Alleviates Adiposity, Hepatic Steatosis, Insulin Resistance, and Inflammation in Diet-Induced Obese Mice
Abstract
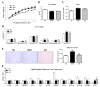
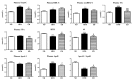
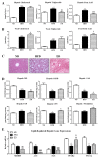
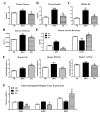
The present study aimed to investigate the molecular mechanisms underlying the anti-obesity effect of flavonoid eriodictyol (ED) supplementation in mice fed with a high-fat diet (HFD). C57BL/6N mice were fed with normal diet (ND), HFD (40 kcal% fat), or HFD + 0.005% (w/w) ED for 16 weeks. In HFD-induced obese mice, dietary ED supplementation significantly alleviated dyslipidemia and adiposity by downregulating the expression of lipogenesis-related genes in white adipose tissue (WAT), while enhancing fecal lipid excretion. ED additionally improved hepatic steatosis and decreased the production of pro-inflammatory cytokines by downregulating the expression of hepatic enzymes and the genes involved in lipogenesis and upregulating the expression of hepatic fatty acid oxidation-related enzymes and genes. In addition, ED improved insulin resistance (IR) by suppressing hepatic gluconeogenesis, enhancing glucose utilization, and modulating the production and release of two incretin hormones, namely gastric inhibitory polypeptide (GIP) and glucagon-like peptide-1 (GLP-1). Taken together, the current findings indicated that ED can protect against diet-induced obesity and related metabolic disturbances, including dyslipidemia, inflammation, fatty liver disease, and IR in diet-induced obese mice.
Keywords: adiposity; eriodictyol; hepatic steatosis; inflammation; insulin resistance; obesity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Seabuckthorn Leaves Extract and Flavonoid Glycosides Extract from Seabuckthorn Leaves Ameliorates Adiposity, Hepatic Steatosis, Insulin Resistance, and Inflammation in Diet-Induced Obesity.Nutrients. 2017 Jun 2;9(6):569. doi: 10.3390/nu9060569. Nutrients. 2017. PMID: 28574484 Free PMC article.
-
Luteolin-Enriched Artichoke Leaf Extract Alleviates the Metabolic Syndrome in Mice with High-Fat Diet-Induced Obesity.Nutrients. 2018 Jul 27;10(8):979. doi: 10.3390/nu10080979. Nutrients. 2018. PMID: 30060507 Free PMC article.
-
Phlorizin Supplementation Attenuates Obesity, Inflammation, and Hyperglycemia in Diet-Induced Obese Mice Fed a High-Fat Diet.Nutrients. 2016 Feb 16;8(2):92. doi: 10.3390/nu8020092. Nutrients. 2016. PMID: 26891322 Free PMC article.
-
Omega-3 fatty acids and adipose tissue biology.Mol Aspects Med. 2018 Dec;64:147-160. doi: 10.1016/j.mam.2018.01.004. Epub 2018 Jan 17. Mol Aspects Med. 2018. PMID: 29329795 Review.
-
Metabolic liver disease of obesity and role of adipose tissue in the pathogenesis of nonalcoholic fatty liver disease.World J Gastroenterol. 2007 Jul 14;13(26):3540-53. doi: 10.3748/wjg.v13.i26.3540. World J Gastroenterol. 2007. PMID: 17659704 Free PMC article. Review.
Cited by
-
Optimized De Novo Eriodictyol Biosynthesis in Streptomyces albidoflavus Using an Expansion of the Golden Standard Toolkit for Its Use in Actinomycetes.Int J Mol Sci. 2023 May 17;24(10):8879. doi: 10.3390/ijms24108879. Int J Mol Sci. 2023. PMID: 37240225 Free PMC article.
-
Association between intake of flavanones and the overweight/obesity and central obesity in children and adolescents: a cross-sectional study from the NHANES database.Front Nutr. 2024 Jul 17;11:1430140. doi: 10.3389/fnut.2024.1430140. eCollection 2024. Front Nutr. 2024. PMID: 39086546 Free PMC article.
-
Protective effect of eriodictyol against hyperglycemia-induced diabetic nephropathy in rats entails antioxidant and anti-inflammatory effects mediated by activating Nrf2.Saudi Pharm J. 2023 Nov;31(11):101817. doi: 10.1016/j.jsps.2023.101817. Epub 2023 Oct 5. Saudi Pharm J. 2023. PMID: 37915829 Free PMC article.
-
Randomized double blind clinical trial evaluating the Ellagic acid effects on insulin resistance, oxidative stress and sex hormones levels in women with polycystic ovarian syndrome.J Ovarian Res. 2021 Jul 31;14(1):100. doi: 10.1186/s13048-021-00849-2. J Ovarian Res. 2021. PMID: 34330312 Free PMC article. Clinical Trial.
-
Associations of the Intake of Individual and Multiple Flavonoids with Metabolic Dysfunction Associated Steatotic Liver Disease in the United States.Nutrients. 2025 Jan 7;17(2):205. doi: 10.3390/nu17020205. Nutrients. 2025. PMID: 39861335 Free PMC article.
References
-
- Kabir M., Catalano K.J., Ananthnarayan S., Kim S.P., van Citters G.W., Dea M.K., Bergman R.N. Molecular evidence supporting the portal theory: A causative link between visceral adiposity and hepatic insulin resistance. Am. J. Physiol. Endocrinol. MeTable. 2005;288:E454–E461. doi: 10.1152/ajpendo.00203.2004. - DOI - PubMed
-
- De Sousa L.M., de Carvalho J.L., da Silva H.C., Lemos T.L., Arriaga A.M., Braz-Filho R., Militão G.C., Silva T.D., Ribeiro P.R., Santiago G.M. New cytotoxic bibenzyl and other constituents from Bauhinia Ungulata L. (Fabaceae) Chem. Biodivers. 2016;13:1630–1635. doi: 10.1002/cbdv.201600058. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

