Deciphering Nuclear Mechanobiology in Laminopathy
- PMID: 30862117
- PMCID: PMC6468464
- DOI: 10.3390/cells8030231
Deciphering Nuclear Mechanobiology in Laminopathy
Abstract
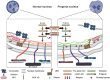
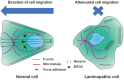
Extracellular mechanical stimuli are translated into biochemical signals inside the cell via mechanotransduction. The nucleus plays a critical role in mechanoregulation, which encompasses mechanosensing and mechanotransduction. The nuclear lamina underlying the inner nuclear membrane not only maintains the structural integrity, but also connects the cytoskeleton to the nuclear envelope. Lamin mutations, therefore, dysregulate the nuclear response, resulting in abnormal mechanoregulations, and ultimately, disease progression. Impaired mechanoregulations even induce malfunction in nuclear positioning, cell migration, mechanosensation, as well as differentiation. To know how to overcome laminopathies, we need to understand the mechanisms of laminopathies in a mechanobiological way. Recently, emerging studies have demonstrated the varying defects from lamin mutation in cellular homeostasis within mechanical surroundings. Therefore, this review summarizes recent findings highlighting the role of lamins, the architecture of nuclear lamina, and their disease relevance in the context of nuclear mechanobiology. We will also provide an overview of the differentiation of cellular mechanics in laminopathy.
Keywords: cytoskeleton; lamin; laminopathy; nucleus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Swift J., Ivanovska I.L., Buxboim A., Harada T., Dingal P.C., Pinter J., Pajerowski J.D., Spinler K.R., Shin J.W., Tewari M., et al. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science. 2013;341:1240104. doi: 10.1126/science.1240104. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

