Effects of minocycline on epiplexus macrophage activation, choroid plexus injury and hydrocephalus development in spontaneous hypertensive rats
- PMID: 30862302
- PMCID: PMC6775580
- DOI: 10.1177/0271678X19836117
Effects of minocycline on epiplexus macrophage activation, choroid plexus injury and hydrocephalus development in spontaneous hypertensive rats
Abstract
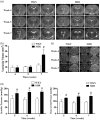
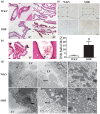
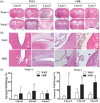
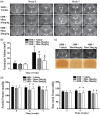
Hydrocephalus has been reported to occur in spontaneous hypertensive rats (SHRs). The purposes of this study were (1) to use T2 magnetic resonance imaging to examine time of onset, (2) to elucidate potential underlying mechanisms and (3) to determine whether minocycline could prevent hydrocephalus development. Ventriculomegaly was evaluated by T2 imaging in SHRs and Wistar-Kyoto rats from weeks 4 to 7 after birth. Brain histology and transmission electron microscopy were used to assess the periventricular and choroid plexus damage. SHRs were also treated with either vehicle or minocycline. We found that hydrocephalus was observed in SHRs but not in Wistar-Kyoto rats. It occurred at seven weeks of age but was not present at four and five weeks. The hydrocephalus was associated with epiplexus cell (macrophage) activation, choroid plexus cell death and damage to the ventricle wall. Treatment with minocycline from week 5 attenuated hydrocephalus development and pathological changes in choroid plexus and ventricular wall at week 7. The current study found that spontaneous hydrocephalus arises at ∼7 weeks in male SHRs. The early development of hydrocephalus (persistent ventricular dilatation) may result from epiplexus cell activation, choroid plexus cell death and periventricular damage, which can be ameliorated by treatment with minocycline.
Keywords: Choroid plexus; epiplexus cells; hydrocephalus; minocycline; spontaneous hypertensive rats.
Figures


Similar articles
-
Prx2 (Peroxiredoxin 2) as a Cause of Hydrocephalus After Intraventricular Hemorrhage.Stroke. 2020 May;51(5):1578-1586. doi: 10.1161/STROKEAHA.119.028672. Epub 2020 Apr 13. Stroke. 2020. PMID: 32279622 Free PMC article.
-
Delayed Minocycline Treatment Ameliorates Hydrocephalus Development and Choroid Plexus Inflammation in Spontaneously Hypertensive Rats.Int J Mol Sci. 2022 Feb 19;23(4):2306. doi: 10.3390/ijms23042306. Int J Mol Sci. 2022. PMID: 35216420 Free PMC article.
-
Minocycline attenuates hydrocephalus and inhibits iron accumulation, ependymal damage and epiplexus cell activation after intraventricular hemorrhage in aged rats.Exp Neurol. 2023 Nov;369:114523. doi: 10.1016/j.expneurol.2023.114523. Epub 2023 Aug 30. Exp Neurol. 2023. PMID: 37652293 Free PMC article.
-
A case report of hydrocephalus due to diffuse villous hyperplasia of the choroid plexus: surgical treatment by combination a flexible videoscope with a rigid endoscope.Childs Nerv Syst. 2023 Aug;39(8):2045-2051. doi: 10.1007/s00381-023-06012-0. Epub 2023 Jul 1. Childs Nerv Syst. 2023. PMID: 37393333 Review.
-
Cellular damage and prevention in childhood hydrocephalus.Brain Pathol. 2004 Jul;14(3):317-24. doi: 10.1111/j.1750-3639.2004.tb00071.x. Brain Pathol. 2004. PMID: 15446588 Free PMC article. Review.
Cited by
-
Prx2 (Peroxiredoxin 2) as a Cause of Hydrocephalus After Intraventricular Hemorrhage.Stroke. 2020 May;51(5):1578-1586. doi: 10.1161/STROKEAHA.119.028672. Epub 2020 Apr 13. Stroke. 2020. PMID: 32279622 Free PMC article.
-
Macrophage activation in stellate ganglia contributes to lung injury-induced arrhythmogenesis in male rats.Acta Physiol (Oxf). 2021 Jun;232(2):e13657. doi: 10.1111/apha.13657. Epub 2021 Apr 14. Acta Physiol (Oxf). 2021. PMID: 33817984 Free PMC article.
-
Neurogenesis and glial impairments in congenital hydrocephalus: insights from a BioGlue-induced fetal lamb model.Fluids Barriers CNS. 2025 Feb 24;22(1):20. doi: 10.1186/s12987-025-00630-3. Fluids Barriers CNS. 2025. PMID: 39994758 Free PMC article.
-
Novel therapeutic modulators of astrocytes for hydrocephalus.Front Mol Neurosci. 2022 Sep 26;15:932955. doi: 10.3389/fnmol.2022.932955. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36226316 Free PMC article. Review.
-
Is choroid plexus growth altered in isolated ventriculomegaly on fetal neuro-ultrasound?Eur Radiol. 2025 Jan;35(1):463-473. doi: 10.1007/s00330-024-10966-3. Epub 2024 Jul 17. Eur Radiol. 2025. PMID: 39014090 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

