Fossorial Damaraland mole rats do not exhibit a blunted hypercapnic ventilatory response
- PMID: 30862308
- PMCID: PMC6451387
- DOI: 10.1098/rsbl.2019.0006
Fossorial Damaraland mole rats do not exhibit a blunted hypercapnic ventilatory response
Abstract
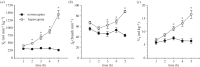
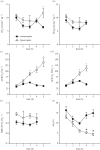
Damaraland mole rats (DMRs, Fukomys damarensis) are a eusocial fossorial species that spend the majority of their life in densely populated underground burrows, in which they likely experience intermittent periods of elevated CO2 (i.e. hypercapnia). The primary physiological response to hypercapnia in most mammals is to increase depth and rate of breathing (i.e. hyperpnoea), but this response is often blunted in species that inhabit hypercapnic environments. In their natural habitat, DMRs putatively experience a gaseous environment ranging from normocapnic (0.1% CO2) to hypercapnic (6.0% CO2) conditions (Roper et al. 2001 J. Zool. 254, 101-107). As such, we hypothesized that DMRs would exhibit blunted hypercapnic ventilatory and metabolic responses, relative to those of non-fossorial rodent species. To test this hypothesis, we exposed awake, freely behaving DMRs to normoxic normocapnia (21% O2, 0% CO2, balance N2) or graded normoxic hypercapnia (21% O2, 0, 2, 5, 7 and 10% CO2, balance N2), and measured ventilation and metabolism using whole-body plethysmography and indirect calorimetry, respectively. We found that ventilation and metabolism were unchanged during prolonged normocapnia, whereas during graded hypercapnia, ventilation was elevated at 2% CO2 and above. As a result, O2 extraction efficiency at the lungs decreased with increasing hyperpnoea. Conversely, metabolic rate did not increase until 10% CO2, presumably due to the metabolic cost of hyperpnoea. Taken together, our results suggest that despite their fossorial lifestyle, DMRs do not exhibit adaptations in their ventilatory or metabolic responses to environmental hypercapnia.
Keywords: hypercapnic metabolic response; hypercapnic ventilatory response; hyperpnoea.
Conflict of interest statement
We have no competing interests.
Figures
Similar articles
-
Neurokinin-1 receptor activation is sufficient to restore the hypercapnic ventilatory response in the Substance P-deficient naked mole-rat.Am J Physiol Regul Integr Comp Physiol. 2020 Apr 1;318(4):R712-R721. doi: 10.1152/ajpregu.00251.2019. Epub 2020 Jan 22. Am J Physiol Regul Integr Comp Physiol. 2020. PMID: 31967860 Free PMC article.
-
Fossorial giant Zambian mole-rats have blunted ventilatory responses to environmental hypoxia and hypercapnia.Comp Biochem Physiol A Mol Integr Physiol. 2020 May;243:110672. doi: 10.1016/j.cbpa.2020.110672. Epub 2020 Feb 4. Comp Biochem Physiol A Mol Integr Physiol. 2020. PMID: 32032753
-
Ventilatory, metabolic, and thermoregulatory responses of Damaraland mole rats to acute and chronic hypoxia.J Comp Physiol B. 2019 Apr;189(2):319-334. doi: 10.1007/s00360-019-01206-y. Epub 2019 Feb 6. J Comp Physiol B. 2019. PMID: 30725174
-
Ventilatory responses to hypoxic and hypercapnic environments in naked mole-rats.Acta Physiol (Oxf). 2023 May;238(1):e13963. doi: 10.1111/apha.13963. Epub 2023 Mar 31. Acta Physiol (Oxf). 2023. PMID: 36924017 Review.
-
Postnatal changes in O2 and CO2 sensitivity in rodents.Respir Physiol Neurobiol. 2020 Jan;272:103313. doi: 10.1016/j.resp.2019.103313. Epub 2019 Oct 15. Respir Physiol Neurobiol. 2020. PMID: 31626974 Review.
Cited by
-
Adenosine and γ-aminobutyric acid partially regulate metabolic and ventilatory responses of Damaraland mole-rats to acute hypoxia.J Exp Biol. 2023 Oct 1;226(19):jeb246186. doi: 10.1242/jeb.246186. Epub 2023 Oct 6. J Exp Biol. 2023. PMID: 37694288 Free PMC article.
-
A New Laboratory Research Model: The Damaraland Mole-rat and Its Managed Care.J Am Assoc Lab Anim Sci. 2024 Nov 1;63(6):683-693. doi: 10.30802/AALAS-JAALAS-24-052. J Am Assoc Lab Anim Sci. 2024. PMID: 39179367 Free PMC article.
-
Hypoxic and hypercapnic burrow conditions lead to downregulation of free triiodothyronine and hematocrit in Ansell's mole-rats (Fukomys anselli).J Comp Physiol B. 2024 Feb;194(1):33-40. doi: 10.1007/s00360-023-01526-0. Epub 2023 Dec 7. J Comp Physiol B. 2024. PMID: 38059996 Free PMC article.
-
Physiological responses to hypoxia are constrained by environmental temperature in heterothermic tenrecs.J Exp Biol. 2023 Mar 15;226(6):jeb245324. doi: 10.1242/jeb.245324. Epub 2023 Mar 29. J Exp Biol. 2023. PMID: 36897570 Free PMC article.
-
Neurokinin-1 receptor activation is sufficient to restore the hypercapnic ventilatory response in the Substance P-deficient naked mole-rat.Am J Physiol Regul Integr Comp Physiol. 2020 Apr 1;318(4):R712-R721. doi: 10.1152/ajpregu.00251.2019. Epub 2020 Jan 22. Am J Physiol Regul Integr Comp Physiol. 2020. PMID: 31967860 Free PMC article.
References
-
- Burnett LE. 1997. The challenges of living in hypoxic and hypercapnic aquatic environments. Environments 37, 633–640.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

