Long-term efficacy and tolerability of TNFα inhibitors in the treatment of non-infectious ocular inflammation: an 8-year prospective surveillance study
- PMID: 30862619
- PMCID: PMC8380906
- DOI: 10.1136/bjophthalmol-2018-312767
Long-term efficacy and tolerability of TNFα inhibitors in the treatment of non-infectious ocular inflammation: an 8-year prospective surveillance study
Abstract
Background/aim: To report the efficacy and tolerability of antitumour necrosis factor-alpha therapy (TNF inhibitors [TNFi]) in the management of non-infectious ocular inflammation, including uveitis and scleritis, in adult patients over an 8-year period.
Materials and methods: This is a prospective cohort study of infliximab and adalimumab in the treatment of non-infectious ocular inflammatory disease. 43 of 85 adult patients on TNFi (34 infliximab, 9 adalimumab) for ≥1 year with non-infectious uveitis or scleritis were followed from 2006 to 2014. Clinical assessments, medication, adverse events and history of steroid rescues were collected at 6 monthly intervals. General quality of life (Short Form Health Survey (SF-36)) and visual quality of life (Vision-related quality of life Core Measure (VCM1)) were assessed annually. Outcome measures included rate of sustained remission, rate of relapse, systemic corticosteroid reduction, adverse events, and VCM1 and SF-36 scores.
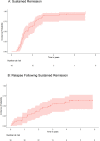
Results: The median time on infliximab was 3.2 years (IQR 4.3) and on adalimumab was 2.4 years (IQR 1.8). Sustained remission was induced in 39 patients (91%) (0.5 per patient year) after a median of 1.2 years on a TNFi. 22 (51%) experienced one relapse, and 5 (12%) had two relapses. 23 (54%) had at least one adverse event; serious adverse events necessitating hospitalisation or cessation of medication occurred in four (9%) patients. 10 patients (23%) switched from the initiation of TNFi, at 1.7 years after starting, to another TNFi or another class of biologic therapy.
Conclusion: TNFi treatment is associated with long-term drug-induced remission of ocular inflammation, visual stability and corticosteroid reduction. Adverse events were common and no new safety signals occurred. Relapse of inflammation occurs in half of the treated population.
Keywords: drugs; immunology; inflammation.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: SMS has participated in advisory boards for AbbVie, outside the submitted work. ADD has been an external advisor for AbbVie, Novartis and Roche, outside the submitted work. ADD and SMS have participated in educational initiatives on behalf of AbbVie. SMS and ADD have received honoraria from AbbVie. All other authors have no competing interests to report.
Figures

References
-
- Nguyen QD, Merrill PT, Jaffe GJ, et al. . Adalimumab for prevention of uveitic flare in patients with inactive non-infectious uveitis controlled by corticosteroids (visual II): a multicentre, double-masked, randomised, placebo-controlled phase 3 trial. Lancet 2016;388:1183–92. 10.1016/S0140-6736(16)31339-3 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
