The COMET (Comparison of Operative versus Monitoring and Endocrine Therapy) trial: a phase III randomised controlled clinical trial for low-risk ductal carcinoma in situ (DCIS)
- PMID: 30862637
- PMCID: PMC6429899
- DOI: 10.1136/bmjopen-2018-026797
The COMET (Comparison of Operative versus Monitoring and Endocrine Therapy) trial: a phase III randomised controlled clinical trial for low-risk ductal carcinoma in situ (DCIS)
Abstract
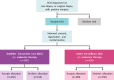
Introduction: Ductal carcinoma in situ (DCIS) is a non-invasive non-obligate precursor of invasive breast cancer. With guideline concordant care (GCC), DCIS outcomes are at least as favourable as some other early stage cancer types such as prostate cancer, for which active surveillance (AS) is a standard of care option. However, AS has not yet been tested in relation to DCIS. The goal of the COMET (Comparison of Operative versus Monitoring and Endocrine Therapy) trial for low-risk DCIS is to gather evidence to help future patients consider the range of treatment choices for low-risk DCIS, from standard therapies to AS. The trial will determine whether there may be some women who do not substantially benefit from current GCC and who could thus be safely managed with AS. This protocol is version 5 (11 July 2018). Any future protocol amendments will be submitted to Quorum Centralised Institutional Review Board/local institutional review boards for approval via the sponsor of the study (Alliance Foundation Trials).
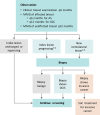
Methods and analysis: COMET is a phase III, randomised controlled clinical trial for patients with low-risk DCIS. The primary outcome is ipsilateral invasive breast cancer rate in women undergoing GCC compared with AS. Secondary objectives will be to compare surgical, oncological and patient-reported outcomes. Patients randomised to the GCC group will undergo surgery as well as radiotherapy when appropriate; those in the AS group will be monitored closely with surgery only on identification of invasive breast cancer. Patients in both the GCC and AS groups will have the option of endocrine therapy. The total planned accrual goal is 1200 patients.
Ethics and dissemination: The COMET trial will be subject to biannual formal review at the Alliance Foundation Data Safety Monitoring Board meetings. Interim analyses for futility/safety will be completed annually, with reporting following Consolidated Standards of Reporting Trials (CONSORT) guidelines for non-inferiority trials.
Trial registration number: NCT02926911; Pre-results.
Keywords: active surveillance; breast cancer; clinical trial; ductal carcinoma in situ; non-invasive; surgery.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Cancer Facts and Figures. Special section: breast carcinoma in situ. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-... (Accessed 15 May 2018).
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
