Broad and Protective Influenza B Virus Neuraminidase Antibodies in Humans after Vaccination and their Clonal Persistence as Plasma Cells
- PMID: 30862743
- PMCID: PMC6414695
- DOI: 10.1128/mBio.00066-19
Broad and Protective Influenza B Virus Neuraminidase Antibodies in Humans after Vaccination and their Clonal Persistence as Plasma Cells
Abstract
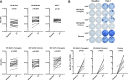
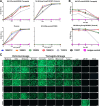
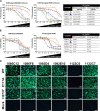
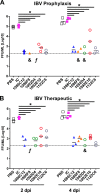
Although most seasonal inactivated influenza vaccines (IIV) contain neuraminidase (NA), the extent and mechanisms of action of protective human NA-specific humoral responses induced by vaccination are poorly resolved. Due to the propensity of influenza virus for antigenic drift and shift and its tendency to elicit predominantly strain-specific antibodies, humanity remains susceptible to waves of new strains of seasonal viruses and is at risk from viruses with pandemic potential for which limited or no immunity may exist. Here we demonstrate that the use of IIV results in increased levels of influenza B virus (IBV) NA-specific serum antibodies. Detailed analysis of the IBV NA B cell response indicates concurrent expansion of IBV NA-specific peripheral blood plasmablasts 7 days after IIV immunization which express monoclonal antibodies with broad and potent antiviral activity against both IBV Victoria and Yamagata lineages and prophylactic and therapeutic activity in mice. These IBV NA-specific B cell clonal lineages persisted in CD138+ long-lived bone marrow plasma cells. These results represent the first demonstration that IIV-induced NA human antibodies can protect and treat influenza virus infection in vivo and suggest that IIV can induce a subset of IBV NA-specific B cells with broad protective potential, a feature that warrants further study for universal influenza vaccine development.IMPORTANCE Influenza virus infections continue to cause substantial morbidity and mortality despite the availability of seasonal vaccines. The extensive genetic variability in seasonal and potentially pandemic influenza strains necessitates new vaccine strategies that can induce universal protection by focusing the immune response on generating protective antibodies against conserved targets such as regions within the influenza neuraminidase protein. We have demonstrated that seasonal immunization stimulates neuraminidase-specific antibodies in humans that are broad and potent in their protection from influenza B virus when tested in mice. These antibodies further persist in the bone marrow, where they are expressed by long-lived antibody-producing cells, referred to here as plasma cells. The significance in our research is the demonstration that seasonal influenza immunization can induce a subset of neuraminidase-specific B cells with broad protective potential, a process that if further studied and enhanced could aid in the development of a universal influenza vaccine.
Keywords: B cell responses; human; influenza vaccines; monoclonal antibodies; neuraminidase.
Copyright © 2019 Piepenbrink et al.
Figures
Similar articles
-
Highly Cross-Reactive and Protective Influenza A Virus H3N2 Hemagglutinin- and Neuraminidase-Specific Human Monoclonal Antibodies.Microbiol Spectr. 2023 Aug 17;11(4):e0472822. doi: 10.1128/spectrum.04728-22. Epub 2023 Jun 15. Microbiol Spectr. 2023. PMID: 37318331 Free PMC article.
-
Dual roles of influenza B virus neuraminidase mRNA vaccine in enhancing cross-lineage protection by supplementing inactivated split vaccination.J Virol. 2025 May 20;99(5):e0229424. doi: 10.1128/jvi.02294-24. Epub 2025 Apr 23. J Virol. 2025. PMID: 40265888 Free PMC article.
-
Vaccination with adjuvanted recombinant neuraminidase induces broad heterologous, but not heterosubtypic, cross-protection against influenza virus infection in mice.mBio. 2015 Mar 10;6(2):e02556. doi: 10.1128/mBio.02556-14. mBio. 2015. PMID: 25759506 Free PMC article.
-
Influenza B virus neuraminidase: a potential target for next-generation vaccines?Expert Rev Vaccines. 2024 Jan-Dec;23(1):39-48. doi: 10.1080/14760584.2023.2290691. Epub 2023 Dec 14. Expert Rev Vaccines. 2024. PMID: 38037386 Review.
-
Contribution of antibody production against neuraminidase to the protection afforded by influenza vaccines.Rev Med Virol. 2012 Jul;22(4):267-79. doi: 10.1002/rmv.1713. Epub 2012 Mar 22. Rev Med Virol. 2012. PMID: 22438243 Free PMC article. Review.
Cited by
-
Neuraminidase antigenic drift of H3N2 clade 3c.2a viruses alters virus replication, enzymatic activity and inhibitory antibody binding.PLoS Pathog. 2020 Jun 29;16(6):e1008411. doi: 10.1371/journal.ppat.1008411. eCollection 2020 Jun. PLoS Pathog. 2020. PMID: 32598381 Free PMC article.
-
Human Antibodies Targeting Influenza B Virus Neuraminidase Active Site Are Broadly Protective.Immunity. 2020 Oct 13;53(4):852-863.e7. doi: 10.1016/j.immuni.2020.08.015. Epub 2020 Sep 24. Immunity. 2020. PMID: 32976769 Free PMC article.
-
Persistence of HIV-1 Env-Specific Plasmablast Lineages in Plasma Cells after Vaccination in Humans.Cell Rep Med. 2020 May 19;1(2):100015. doi: 10.1016/j.xcrm.2020.100015. Cell Rep Med. 2020. PMID: 32577626 Free PMC article.
-
The Human Antibody Response to the Influenza Virus Neuraminidase Following Infection or Vaccination.Vaccines (Basel). 2021 Aug 2;9(8):846. doi: 10.3390/vaccines9080846. Vaccines (Basel). 2021. PMID: 34451971 Free PMC article. Review.
-
Anti-neuraminidase immunity in the combat against influenza.Expert Rev Vaccines. 2024 Jan-Dec;23(1):474-484. doi: 10.1080/14760584.2024.2343689. Epub 2024 Apr 23. Expert Rev Vaccines. 2024. PMID: 38632930 Free PMC article. Review.
References
-
- Centers for Disease Control and Prevention. 2011. Influenza-associated pediatric deaths—United States, September 2010–August 2011. MMWR Morb Mortal Wkly Rep 60:1233–1238. - PubMed
-
- Skowronski DM, Chambers C, De Serres G, Sabaiduc S, Winter AL, Dickinson JA, Gubbay JB, Fonseca K, Drews SJ, Charest H, Martineau C, Krajden M, Petric M, Bastien N, Li Y. 2017. Age-related differences in influenza B infection by lineage in a community-based sentinel system, 2010–2011 to 2015–2016, Canada. J Infect Dis 216:697–702. doi:10.1093/infdis/jix393. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

