Noninvasive sub-organ ultrasound stimulation for targeted neuromodulation
- PMID: 30862827
- PMCID: PMC6414607
- DOI: 10.1038/s41467-019-08750-9
Noninvasive sub-organ ultrasound stimulation for targeted neuromodulation
Erratum in
-
Author Correction: Noninvasive sub-organ ultrasound stimulation for targeted neuromodulation.Nat Commun. 2020 Mar 9;11(1):1336. doi: 10.1038/s41467-020-15011-7. Nat Commun. 2020. PMID: 32152308 Free PMC article.
Abstract
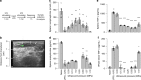
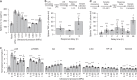
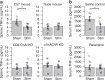
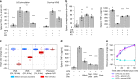
Tools for noninvasively modulating neural signaling in peripheral organs will advance the study of nerves and their effect on homeostasis and disease. Herein, we demonstrate a noninvasive method to modulate specific signaling pathways within organs using ultrasound (U/S). U/S is first applied to spleen to modulate the cholinergic anti-inflammatory pathway (CAP), and US stimulation is shown to reduce cytokine response to endotoxin to the same levels as implant-based vagus nerve stimulation (VNS). Next, hepatic U/S stimulation is shown to modulate pathways that regulate blood glucose and is as effective as VNS in suppressing the hyperglycemic effect of endotoxin exposure. This response to hepatic U/S is only found when targeting specific sub-organ locations known to contain glucose sensory neurons, and both molecular (i.e. neurotransmitter concentration and cFOS expression) and neuroimaging results indicate US induced signaling to metabolism-related hypothalamic sub-nuclei. These data demonstrate that U/S stimulation within organs provides a new method for site-selective neuromodulation to regulate specific physiological functions.
Conflict of interest statement
V.C., Y.F., I.H., P.F., K.W., S.K., J.G., W.R., T.-J.K., J.R., C.B., S.J., J.A. and C.P. are employees of General Electric and declare that GE has filed US and international patent applications describing methods, devices, and systems for precision organ-based U/S neuromodulation. T.T., A.M.K., T.R.C., S.Z., K.J.T. and S.S.C. declare that GE provided funds to support their work associated with this manuscript. All other authors declare no competing interests.
Figures



Comment in
-
Peripheral Focused Ultrasound Stimulation (pFUS): New Competitor in Pharmaceutical Markets?SLAS Technol. 2019 Aug;24(4):448-452. doi: 10.1177/2472630319849383. Epub 2019 Jun 17. SLAS Technol. 2019. PMID: 31226243 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

