Characterization, optimization, and in vitro evaluation of Technetium-99m-labeled niosomes
- PMID: 30863048
- PMCID: PMC6391155
- DOI: 10.2147/IJN.S184912
Characterization, optimization, and in vitro evaluation of Technetium-99m-labeled niosomes
Abstract
Background and purpose: Niosomes are nonionic surfactant-based vesicles that exhibit certain unique features which make them favorable nanocarriers for sustained drug delivery in cancer therapy. Biodistribution studies are critical in assessing if a nanocarrier system has preferential accumulation in a tumor by enhanced permeability and retention effect. Radiolabeling of nanocarriers with radioisotopes such as Technetium-99m (99mTc) will allow for the tracking of the nanocarrier noninvasively via nuclear imaging. The purpose of this study was to formulate, characterize, and optimize 99mTc-labeled niosomes.
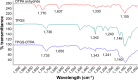
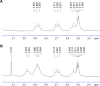
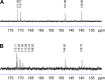
Methods: Niosomes were prepared from a mixture of sorbitan monostearate 60, cholesterol, and synthesized D-α-tocopherol polyethylene glycol 1000 succinate-diethylenetriaminepentaacetic acid (synthesis confirmed by 1H and 13C nuclear magnetic resonance spectroscopy). Niosomes were radiolabeled by surface chelation with reduced 99mTc. Parameters affecting the radiolabeling efficiency such as concentration of stannous chloride (SnCl2·H2O), pH, and incubation time were evaluated. In vitro stability of radiolabeled niosomes was studied in 0.9% saline and human serum at 37°C for up to 8 hours.
Results: Niosomes had an average particle size of 110.2±0.7 nm, polydispersity index of 0.229±0.008, and zeta potential of -64.8±1.2 mV. Experimental data revealed that 30 µg/mL of SnCl2·H2O was the optimal concentration of reducing agent required for the radiolabeling process. The pH and incubation time required to obtain high radiolabeling efficiency was pH 5 and 15 minutes, respectively. 99mTc-labeled niosomes exhibited high radiolabeling efficiency (>90%) and showed good in vitro stability for up to 8 hours.
Conclusion: To our knowledge, this is the first study published on the surface chelation of niosomes with 99mTc. The formulated 99mTc-labeled niosomes possessed high radiolabeling efficacy, good stability in vitro, and show good promise for potential use in nuclear imaging in the future.
Keywords: drug delivery; formulation; nanocarriers; nanotechnology; nuclear imaging; radiolabeling.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures





References
-
- Williams EC, Toomey R, Alcantar N. Controlled release niosome embedded chitosan system: Effect of crosslink mesh dimensions on drug release. J Biomed Mater Res A. 2012;100A(12):3296–3303. - PubMed
-
- Abdelkader H, Alani AW, Alany RG. Recent advances in non-ionic surfactant vesicles (niosomes): self-assembly, fabrication, characterization, drug delivery applications and limitations. Drug Deliv. 2014;21(2):87–100. - PubMed
-
- Marianecci C, di Marzio L, Rinaldi F, et al. Niosomes from 80s to present: the state of the art. Adv Colloid Interface Sci. 2014;205:187–206. - PubMed
-
- Fathalla D, Abdel-Mageed A, Abdel-Hamid F, In-Vitro AM. In-vitro and in-vivo evaluation of niosomal gel containing aceclofenac for sustained drug delivery. Int J Pharm Sci Res. 2014;1(1):1–11.
-
- Vikbjerg AF, Andresen TL, Jørgensen K, Mu H, Xu X. Oxidative stability of liposomes composed of docosahexaenoic acid-containing phospholipids. J Am Oil Chem Soc. 2007;84(7):631–637.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

