Strategic approach to developing a self-microemulsifying drug delivery system to enhance antiplatelet activity and bioavailability of ticagrelor
- PMID: 30863054
- PMCID: PMC6391151
- DOI: 10.2147/IJN.S190426
Strategic approach to developing a self-microemulsifying drug delivery system to enhance antiplatelet activity and bioavailability of ticagrelor
Abstract
Background: Ticagrelor (TCG) is used to inhibit platelet aggregation in patients with acute coronary syndrome, but its poor solubility and low bioavailability limit its in vivo efficacy. The purpose of this study was to manufacture an optimized TCG-loaded self-microemulsifying drug delivery system (SMEDDS) to enhance the oral bioavailability and antiplatelet activity of TCG.
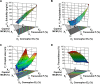
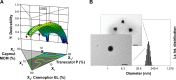
Materials and methods: Solubility and emulsification tests were conducted to determine the most suitable oils, surfactants, and cosurfactants. Scheffé's mixture design was applied to optimize the percentage of each component applied in the SMEDDS formulation to achieve optimal physical characteristics, ie, high solubility of TCG in SMEDDS, small droplet size, low precipitation, and high transmittance.
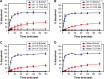
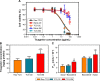
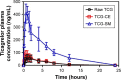
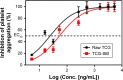
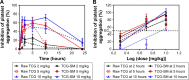
Results: The optimized TCG-loaded SMEDDS (TCG-SM) formulation composed of 10.0% Capmul MCM (oil), 53.8% Cremophor EL (surfactant), and 36.2% Transcutol P (cosurfactant) significantly improving the dissolution of TCG in various media compared with TCG in Brilinta® (commercial product). TCG-SM exhibited higher cellular uptake and permeability in Caco-2 cells than raw TCG suspension. In pharmacokinetic studies in rats, TCG-SM exhibited higher oral bioavailability with 5.7 and 6.4 times higher area under the concentration-time curve and maximum plasma concentration, respectively, than a raw TCG suspension. Antiplatelet activity studies exhibited that the TCG-SM formulation showed significantly improved inhibition of platelet aggregation compared with raw TCG at the same dose of TCG. And, a 10 mg/kg dose of raw TCG suspension and a 5 mg/kg dose of TCG-SM had a similar area under the inhibitory curve (907.0%±408.8% and 907.8%±200.5%⋅hours, respectively) for antiplatelet activity.
Conclusion: These results suggest that the developed TCG-SM could be successfully used as an efficient method to achieve the enhanced antiplatelet activity and bioavailability of TCG.
Keywords: SMEDDS; antiplatelet activity; bioavailability; optimization; platelet aggregation; ticagrelor.
Conflict of interest statement
Disclosure Mr Gi-Ho Son and Mr Ki-Hyun Bang are employed by Korea United Pharmaceutical Co. Ltd., Sejong, Republic of Korea. Mr Sung-Hoon Jeon is employed by SamA Pharmaceutical Co. Ltd., Suwon, Republic of Korea. The authors did not receive grants/funds from these affiliations for this study. The authors report no other conflicts of interest in this work.
Figures



Similar articles
-
Statistical approach for solidifying ticagrelor loaded self-microemulsifying drug delivery system with enhanced dissolution and oral bioavailability.Mater Sci Eng C Mater Biol Appl. 2019 Nov;104:109980. doi: 10.1016/j.msec.2019.109980. Epub 2019 Jul 16. Mater Sci Eng C Mater Biol Appl. 2019. PMID: 31500011
-
Pharmacokinetic/Pharmacodynamic Modeling To Predict the Antiplatelet Effect of the Ticagrelor-Loaded Self-Microemulsifying Drug Delivery System in Rats.Mol Pharm. 2020 Apr 6;17(4):1079-1089. doi: 10.1021/acs.molpharmaceut.9b00964. Epub 2020 Feb 24. Mol Pharm. 2020. PMID: 32053381
-
Development and evaluation of TPGS/PVA-based nanosuspension for enhancing dissolution and oral bioavailability of ticagrelor.Int J Pharm. 2020 May 15;581:119287. doi: 10.1016/j.ijpharm.2020.119287. Epub 2020 Mar 31. Int J Pharm. 2020. PMID: 32243963
-
Self microemulsifying drug delivery system of lurasidone hydrochloride for enhanced oral bioavailability by lymphatic targeting: In vitro, Caco-2 cell line and in vivo evaluation.Eur J Pharm Sci. 2019 Oct 1;138:105027. doi: 10.1016/j.ejps.2019.105027. Epub 2019 Aug 1. Eur J Pharm Sci. 2019. PMID: 31377133
-
Development and optimization of a self-microemulsifying drug delivery system for atorvastatin calcium by using D-optimal mixture design.Int J Nanomedicine. 2015 Jun 5;10:3865-77. doi: 10.2147/IJN.S83520. eCollection 2015. Int J Nanomedicine. 2015. PMID: 26089663 Free PMC article.
Cited by
-
Optimization of a Cefuroxime Axetil-Loaded Liquid Self-Nanoemulsifying Drug Delivery System: Enhanced Solubility, Dissolution and Caco-2 Cell Uptake.Pharmaceutics. 2022 Apr 1;14(4):772. doi: 10.3390/pharmaceutics14040772. Pharmaceutics. 2022. PMID: 35456606 Free PMC article.
-
Synthesis and evaluation of chitosan based controlled release nanoparticles for the delivery of ticagrelor.Des Monomers Polym. 2022 Mar 20;25(1):55-63. doi: 10.1080/15685551.2022.2054117. eCollection 2022. Des Monomers Polym. 2022. PMID: 35341118 Free PMC article.
-
Drug Repositioning For Allosteric Modulation of VIP and PACAP Receptors.Front Endocrinol (Lausanne). 2021 Nov 18;12:711906. doi: 10.3389/fendo.2021.711906. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34867774 Free PMC article.
-
Systemic Design and Evaluation of Ticagrelor-Loaded Nanostructured Lipid Carriers for Enhancing Bioavailability and Antiplatelet Activity.Pharmaceutics. 2019 May 8;11(5):222. doi: 10.3390/pharmaceutics11050222. Pharmaceutics. 2019. PMID: 31071977 Free PMC article.
-
Crosslinked PVA-g-poly(AMPS) Nanogels for Enhanced Solubility and Dissolution of Ticagrelor: Synthesis, Characterization, and Toxicity Evaluation.ACS Omega. 2024 May 2;9(19):21401-21415. doi: 10.1021/acsomega.4c01721. eCollection 2024 May 14. ACS Omega. 2024. PMID: 38764664 Free PMC article.
References
-
- Husted S, Emanuelsson H, Heptinstall S, Sandset PM, Wickens M, Peters G. Pharmacodynamics, pharmacokinetics, and safety of the oral reversible P2Y12 antagonist AZD6140 with aspirin in patients with atherosclerosis: a double-blind comparison to clopidogrel with aspirin. Eur Heart J. 2006;27(9):1038–1047. - PubMed
-
- Teng R, Oliver S, Hayes MA, Butler K. Absorption, distribution, metabolism, and excretion of ticagrelor in healthy subjects. Drug Metab Dispos. 2010;38(9):1514–1521. - PubMed
-
- van Giezen JJ, Humphries RG. Preclinical and clinical studies with selective reversible direct P2Y12 antagonists. Semin Thromb Hemost. 2005;31(02):195–204. - PubMed
-
- Wijeyeratne YD, Joshi R, Heptinstall S. Ticagrelor: a P2Y12 antagonist for use in acute coronary syndromes. Expert Rev Clin Pharmacol. 2012;5(3):257–269. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

