Searching for Bacteria in Neural Tissue From Amyotrophic Lateral Sclerosis
- PMID: 30863279
- PMCID: PMC6399391
- DOI: 10.3389/fnins.2019.00171
Searching for Bacteria in Neural Tissue From Amyotrophic Lateral Sclerosis
Abstract
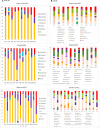
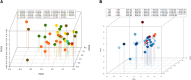
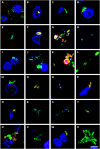
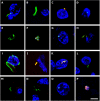
Despite great efforts in the investigation, the exact etiology of amyotrophic lateral sclerosis (ALS) is a matter of intensive research. We recently advanced the idea that ALS might be caused by fungal infection. Indeed, fungal yeast and hyphal structures can be directly visualized in neural tissue of ALS patients, and a number of fungal species have been identified in the central nervous system (CNS). In the present work, we tested the possibility that bacterial infections can accompany these mycoses. Our findings establish the presence of bacterial DNA in different regions of the CNS from all ALS patients examined. Specifically, we used PCR and next generation sequencing (NGS) to precisely determine the bacterial species present in ALS tissue. Consistent with these findings, immunohistochemistry analysis of CNS sections using specific anti-bacterial antibodies identified prokaryotic cells in neural tissue. Finally, we assayed for the repeat expansion of the hexanucleotide repeat GGGGCC in C9orf72, which is considered the most common genetic cause of ALS in patients, using DNA extracted from ALS CNS tissue. We failed to find this repeated sequence in any of the eleven patients analyzed. Our results indicate that bacterial DNA and prokaryotic cells are present in CNS tissue, leading to the concept that both fungal and bacterial infections coexist in patients with ALS. These observations lay the groundwork for the use of appropriate therapies to eradicate the polymicrobial infections in ALS.
Keywords: amyotrophic lateral sclerosis; neurodegenerative disease; next generation sequencing; polymicrobial infection; repeat expansion C9orf72.
Figures