Resolvin D2 Induces Resolution of Periapical Inflammation and Promotes Healing of Periapical Lesions in Rat Periapical Periodontitis
- PMID: 30863409
- PMCID: PMC6399419
- DOI: 10.3389/fimmu.2019.00307
Resolvin D2 Induces Resolution of Periapical Inflammation and Promotes Healing of Periapical Lesions in Rat Periapical Periodontitis
Abstract
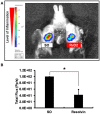
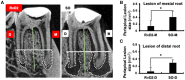
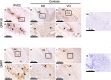
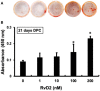
Periapical periodontitis results from pulpal infection leading to pulpal necrosis and resorption of periapical bone. The current treatment is root canal therapy, which attempts to eliminate infection and necrotic tissue. But, in some cases periapical inflammation doesn't resolve even after treatment. Resolvins belongs to a large family of specialized pro-resolving lipid mediators that actively resolves inflammation signaling via specific receptors. Resolvin D2 (RvD2), a metabolite of docosahexaenoic acid (DHA), was tested as an intracanal medicament in rats in vivo. Mechanism was evaluated in rat primary dental pulp cells (DPCs) in vitro. The results demonstrate that RvD2 reduces inflammatory cell infiltrate, periapical lesion size, and fosters pulp like tissue regeneration and healing of periapical lesion. RvD2 enhanced expression of its receptor, GPR18, dentin matrix acidic phosphoprotein 1 (DMP1) and mineralization in vivo and in vitro. Moreover, RvD2 induces phosphorylation of Stat3 transcription factor in dental pulp cells. We conclude that intracanal treatment with RvD2 resolves inflammation and promoting calcification around root apex and healing of periapical bone lesions. The data suggest that RvD2 induces active resolution of inflammation with pulp-like tissue regeneration after root canal infection and thus maybe suitable for treating periapical lesions.
Keywords: DMP1; calcification; periapical lesion; periapical periodontitis; resolution of periapical inflammation; resolvin D2.
Figures



Similar articles
-
Resolvin D2-induced reparative dentin and pulp stem cells after pulpotomy in a rat model.Heliyon. 2024 Jul 6;10(13):e34206. doi: 10.1016/j.heliyon.2024.e34206. eCollection 2024 Jul 15. Heliyon. 2024. PMID: 39091941 Free PMC article.
-
Wound healing of apical tissues after root canal therapy: a long-term clinical, radiographic, and histopathologic observation study.Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009 Oct;108(4):609-21. doi: 10.1016/j.tripleo.2009.05.028. Epub 2009 Aug 28. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009. PMID: 19716731
-
Resolvin D2 Restrains Th1 Immunity and Prevents Alveolar Bone Loss in Murine Periodontitis.Front Immunol. 2018 Apr 25;9:785. doi: 10.3389/fimmu.2018.00785. eCollection 2018. Front Immunol. 2018. PMID: 29922275 Free PMC article.
-
Nonsurgical root canal therapy of large cyst-like inflammatory periapical lesions and inflammatory apical cysts.J Endod. 2009 May;35(5):607-15. doi: 10.1016/j.joen.2009.02.012. J Endod. 2009. PMID: 19410070 Review.
-
Inflammatory Response Mechanisms of the Dentine-Pulp Complex and the Periapical Tissues.Int J Mol Sci. 2021 Feb 2;22(3):1480. doi: 10.3390/ijms22031480. Int J Mol Sci. 2021. PMID: 33540711 Free PMC article. Review.
Cited by
-
Specialized Pro-Resolving Lipid Mediators: Endogenous Roles and Pharmacological Activities in Infections.Molecules. 2023 Jun 27;28(13):5032. doi: 10.3390/molecules28135032. Molecules. 2023. PMID: 37446699 Free PMC article. Review.
-
Design and Synthesis of Benzene Congeners of Resolvin E2, a Proresolving Lipid Mediator, as Its Stable Equivalents.ACS Med Chem Lett. 2020 Mar 25;11(4):479-484. doi: 10.1021/acsmedchemlett.9b00596. eCollection 2020 Apr 9. ACS Med Chem Lett. 2020. PMID: 32292553 Free PMC article.
-
[Akt2 inhibitor promotes M2 macrophage polarization in rats with periapical inflammation by reducing miR-155-5p expression].Nan Fang Yi Ke Da Xue Xue Bao. 2023 Apr 20;43(4):568-576. doi: 10.12122/j.issn.1673-4254.2023.04.09. Nan Fang Yi Ke Da Xue Xue Bao. 2023. PMID: 37202192 Free PMC article. Chinese.
-
The resolvin D2 and omega-3 polyunsaturated fatty acid as a new possible therapeutic approach for inflammatory bowel diseases.Sci Rep. 2024 Nov 20;14(1):28698. doi: 10.1038/s41598-024-80051-8. Sci Rep. 2024. PMID: 39562789 Free PMC article.
-
Therapeutic Exploitation of GPR18: Beyond the Cannabinoids?J Med Chem. 2020 Dec 10;63(23):14216-14227. doi: 10.1021/acs.jmedchem.0c00926. Epub 2020 Sep 23. J Med Chem. 2020. PMID: 32914978 Free PMC article. Review.
References
-
- Sundqvist G. Bacteriological Studies of Necrotic Dental Pulps. PhD Dissertation, Department of Oral Microbiology, Umea University, Sweden: (1976).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

