Reversal of New Onset Type 1 Diabetes by Oral Salmonella-Based Combination Therapy and Mediated by Regulatory T-Cells in NOD Mice
- PMID: 30863412
- PMCID: PMC6400227
- DOI: 10.3389/fimmu.2019.00320
Reversal of New Onset Type 1 Diabetes by Oral Salmonella-Based Combination Therapy and Mediated by Regulatory T-Cells in NOD Mice
Abstract
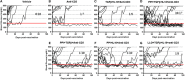
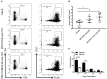
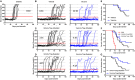
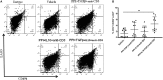
Autoimmune diseases such as type 1 diabetes (T1D) involve the loss of regulatory mechanisms resulting in increased tissue-specific cytotoxicity. The result is destruction of pancreatic insulin-producing β-cells and loss of glucose homeostasis. We are developing a novel oral vaccine using live attenuated Salmonella to deliver TGFβ, IL10, and the diabetic autoantigen preproinsulin combined with low-doses of anti-CD3 mAb. Here we show that oral administration of Salmonella-based anti-CD3 mAb combined therapy reverses new-onset T1D in non-obese diabetic (NOD) mice. The therapeutic effect of the combined therapy was associated with induction of immune suppressive CD4+CD25+Foxp3+ Treg and CD4+CD49b+LAG3+ Tr1 cells. In adoptive transfer experiments, adding or depleting Treg or Tr1 cells indicated that both are important for preventing diabetes in combined therapy-treated mice, but that Tr1 cells may have a more central role. Furthermore, induced Tr1 cells were found to be antigen-specific responding to peptide stimulation by secreting tolerance inducing IL10. These preclinical data demonstrate a role for Treg and Tr1 cells in combined therapy-mediated induction of tolerance in NOD mice. These results also demonstrate the potential of oral Salmonella-based combined therapy in the treatment of early T1D.
Keywords: Salmonella; Tr1-cells; Treg-cells; immunomodulators; immunotherapy; oral vaccine; type 1 diabetes.
Figures

Similar articles
-
Oral Salmonella msbB Mutant as a Carrier for a Salmonella-Based Vaccine for Prevention and Reversal of Type 1 Diabetes.Front Immunol. 2021 May 24;12:667897. doi: 10.3389/fimmu.2021.667897. eCollection 2021. Front Immunol. 2021. PMID: 34108968 Free PMC article.
-
Factors affecting Salmonella-based combination immunotherapy for prevention of type 1 diabetes in non-obese diabetic mice.Vaccine. 2018 Dec 18;36(52):8008-8018. doi: 10.1016/j.vaccine.2018.10.101. Epub 2018 Nov 8. Vaccine. 2018. PMID: 30416020
-
An oral vaccine for type 1 diabetes based on live attenuated Salmonella.Vaccine. 2014 Apr 25;32(20):2300-7. doi: 10.1016/j.vaccine.2014.02.070. Epub 2014 Mar 12. Vaccine. 2014. PMID: 24631074
-
CD3 antibody treatment stimulates the functional capability of regulatory T cells.Novartis Found Symp. 2003;252:279-86; discussion 286-90. Novartis Found Symp. 2003. PMID: 14609225 Review.
-
CD3 monoclonal antibodies: a first step towards operational immune tolerance in the clinic.Rev Diabet Stud. 2012 Winter;9(4):372-81. doi: 10.1900/RDS.2012.9.372. Epub 2012 Dec 28. Rev Diabet Stud. 2012. PMID: 23804274 Free PMC article. Review.
Cited by
-
Gαz-independent and -dependent Improvements With EPA Supplementation on the Early Type 1 Diabetes Phenotype of NOD Mice.J Endocr Soc. 2024 May 21;8(7):bvae100. doi: 10.1210/jendso/bvae100. eCollection 2024 May 23. J Endocr Soc. 2024. PMID: 38831864 Free PMC article.
-
Salmonella-Based Vaccine: A Promising Strategy for Type 1 Diabetes.Vaccines (Basel). 2025 Apr 14;13(4):405. doi: 10.3390/vaccines13040405. Vaccines (Basel). 2025. PMID: 40333284 Free PMC article. Review.
-
IL-27 Regulated CD4+IL-10+ T Cells in Experimental Sjögren Syndrome.Front Immunol. 2020 Aug 11;11:1699. doi: 10.3389/fimmu.2020.01699. eCollection 2020. Front Immunol. 2020. PMID: 32849596 Free PMC article.
-
Changes in the gut microbiota of NOD mice in response to an oral Salmonella-based vaccine against type 1 diabetes.PLoS One. 2023 May 24;18(5):e0285905. doi: 10.1371/journal.pone.0285905. eCollection 2023. PLoS One. 2023. PMID: 37224176 Free PMC article.
-
Oral Salmonella msbB Mutant as a Carrier for a Salmonella-Based Vaccine for Prevention and Reversal of Type 1 Diabetes.Front Immunol. 2021 May 24;12:667897. doi: 10.3389/fimmu.2021.667897. eCollection 2021. Front Immunol. 2021. PMID: 34108968 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

