A novel therapeutic approach to colorectal cancer in diabetes: role of metformin and rapamycin
- PMID: 30863490
- PMCID: PMC6407684
- DOI: 10.18632/oncotarget.26641
A novel therapeutic approach to colorectal cancer in diabetes: role of metformin and rapamycin
Erratum in
-
Correction: A novel therapeutic approach to colorectal cancer in diabetes: role of metformin and rapamycin.Oncotarget. 2019 Nov 5;10(60):6538-6539. doi: 10.18632/oncotarget.27298. eCollection 2019 Nov 5. Oncotarget. 2019. PMID: 31741717 Free PMC article.
Abstract
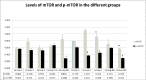
The link between colorectal cancer (CRC), diabetes mellitus (DM) and inflammation is well established, and polytherapy, including rapamycin, has been adopted. This study is a novel approach that aimed at assessing the effect of a combination therapy of metformin and rapamycin on the control or prevention of CRC in diabetic animals, in presence or absence of probiotics. Fifty NOD/SCIDs male mice developed xenograft by inoculating HCT116 cells. They were equally divided into diabetics (induced by Streptozotocin) and non-diabetics. Metformin was given in drinking water, whereas rapamycin was administered via intra-peritoneal injections. Probiotics were added to the double therapy two weeks before the sacrifice. Assessment was performed by clinical observation, histological analysis, Reactive oxygen species (ROS) activities and molecular analysis of Interleukin 3 and 6, Tumor Necrosis Factor alpha, AMP-activated protein Kinase and the mammalian target of rapamycin. Decreases in the level of tumorigenesis resulted, to various extents, with the different treatment regimens. The combination of rapamycin and metformin had no significant result, however, after adding probiotics to the combination, there was a marked delay in tumor formation and reduction of its size, suppression of ROS and a decrease in inflammatory cytokines as well as an inhibition of phosphorylated mTOR. Existing evidence clearly supports the use of rapamycin and metformin especially in the presence of probiotics. It also highlighted the possible mechanism of action of the 2 drugs through AMPK and mTOR signaling pathways and offered preliminary data on the significant role of probiotics in the combination. Further investigation to clarify the exact role of probiotics and decipher in more details the involved pathways is needed.
Keywords: colorectal cancer; diabetes mellitus; inflammatory cytokines; mTOR; probiotics.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare that there is no conflict of interest regarding the publication of this article.
Figures















References
-
- Cannata D, Fierz Y, Vijayakumar A, LeRoith D. Type 2 diabetes and cancer: what is the connection? Mount Sinai Journal of Medicine: A Journal of Translational and Personalized Medicine. 2010;77:197–213. - PubMed
-
- Fischbach S, Gittes GK. The Role of TGF-[Beta] Signaling in [Beta]-Cell Dysfunction and Type 2 Diabetes: A Review. Journal of Cytology & Histology. 2014;5:1.
LinkOut - more resources
Full Text Sources
Miscellaneous

