An overview of fish bioacoustics and the impacts of anthropogenic sounds on fishes
- PMID: 30864159
- PMCID: PMC6849755
- DOI: 10.1111/jfb.13948
An overview of fish bioacoustics and the impacts of anthropogenic sounds on fishes
Abstract
Fishes use a variety of sensory systems to learn about their environments and to communicate. Of the various senses, hearing plays a particularly important role for fishes in providing information, often from great distances, from all around these animals. This information is in all three spatial dimensions, often overcoming the limitations of other senses such as vision, touch, taste and smell. Sound is used for communication between fishes, mating behaviour, the detection of prey and predators, orientation and migration and habitat selection. Thus, anything that interferes with the ability of a fish to detect and respond to biologically relevant sounds can decrease survival and fitness of individuals and populations. Since the onset of the Industrial Revolution, there has been a growing increase in the noise that humans put into the water. These anthropogenic sounds are from a wide range of sources that include shipping, sonars, construction activities (e.g., wind farms, harbours), trawling, dredging and exploration for oil and gas. Anthropogenic sounds may be sufficiently intense to result in death or mortal injury. However, anthropogenic sounds at lower levels may result in temporary hearing impairment, physiological changes including stress effects, changes in behaviour or the masking of biologically important sounds. The intent of this paper is to review the potential effects of anthropogenic sounds upon fishes, the potential consequences for populations and ecosystems and the need to develop sound exposure criteria and relevant regulations. However, assuming that many readers may not have a background in fish bioacoustics, the paper first provides information on underwater acoustics, with a focus on introducing the very important concept of particle motion, the primary acoustic stimulus for all fishes, including elasmobranchs. The paper then provides background material on fish hearing, sound production and acoustic behaviour. This is followed by an overview of what is known about effects of anthropogenic sounds on fishes and considers the current guidelines and criteria being used world-wide to assess potential effects on fishes. Most importantly, the paper provides the most complete summary of the effects of anthropogenic noise on fishes to date. It is also made clear that there are currently so many information gaps that it is almost impossible to reach clear conclusions on the nature and levels of anthropogenic sounds that have potential to cause changes in animal behaviour, or even result in physical harm. Further research is required on the responses of a range of fish species to different sound sources, under different conditions. There is a need both to examine the immediate effects of sound exposure and the longer-term effects, in terms of fitness and likely impacts upon populations.
Keywords: behaviour; criteria; effects; guidelines; hearing; sound.
© 2019 The Authors. Journal of Fish Biology published by John Wiley & Sons Ltd on behalf of The Fisheries Society of the British Isles.
Figures

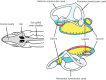
 , The otolith organs,
, The otolith organs,  , the semicircular canals (enlarged areas are the ampullae regions that contain the sensory cells);
, the semicircular canals (enlarged areas are the ampullae regions that contain the sensory cells);  , the dense calcarious otolith lying in close proximity to the sensory epithelium (
, the dense calcarious otolith lying in close proximity to the sensory epithelium ( ). Also see Figure 4. Fig. © 2018 Anthony D. Hawkins, all rights reserved
). Also see Figure 4. Fig. © 2018 Anthony D. Hawkins, all rights reserved
 ). The saccular chamber is filled with perilymph and contains the otolith (
). The saccular chamber is filled with perilymph and contains the otolith ( ), which lies close to the sensory hair cells of the epithelium (macula). The hair cells are innervated by the eighth cranial nerve. Fig. © 2018 Anthony D. Hawkins, all rights reserved
), which lies close to the sensory hair cells of the epithelium (macula). The hair cells are innervated by the eighth cranial nerve. Fig. © 2018 Anthony D. Hawkins, all rights reserved
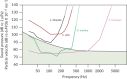
 , The approximate dividing lines between orientation groups)
, The approximate dividing lines between orientation groups)Comment in
-
Noisy waters.J Fish Biol. 2019 May;94(5):691. doi: 10.1111/jfb.13985. J Fish Biol. 2019. PMID: 31074025 No abstract available.
References
-
- Amorim, M. C. P. , Vasconcelos, R. O. , Bolgan, M. , Pedroso, S. S. , & Fonseca, P. J. (2018). Acoustic communication in marine shallow waters: Testing the acoustic adaptive hypothesis in sand gobies. The Journal of Experimental Biology, 221, jeb183681. - PubMed
-
- Amoser, S. , Wysocki, L. E. , & Ladich, F. (2004). Noise emission during the first powerboat race in an alpine lake and potential impact on fish communities. The Journal of the Acoustical Society of America, 116, 3789–3797. - PubMed
-
- Andersson, M. H. , Andersson, S. , Ahlsen, J. , Andersoson, B. L. , Hammar, J. , Persson, L. K. , Pihl, J. , Sigray, P. & Wisstrom, A. (2017). A framework for regulating underwater noise during pile driving. A technical Vindal report. Stockholm: Environmental Protection agency, Stockholm, Sweden. http://www.tethys.pnnl.gov/sites/default/files/publications/Andersson-et...
-
- Bass, A. H. , & Ladich, F. (2008). Vocal‐acoustic communication: From neurons to brain In Webb J. F., Fay R. R., & Popper A. N. (Eds.), Fish bioacoustics (pp. 253–278). New York, NY: Springer Science+Business Media, LLC.
-
- Benhaïm, D. , Péan, S. , Lucas, G. , Blanc, N. , Chatain, B. , & Bégout, M.‐L. (2012). Early life behavioural differences in wild caught and domesticated sea bass (Dicentrarchus labrax). Applied Animal Behaviour Science, 141, 79–90.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
