The tandem zinc finger RNA binding domain of members of the tristetraprolin protein family
- PMID: 30864256
- PMCID: PMC6570553
- DOI: 10.1002/wrna.1531
The tandem zinc finger RNA binding domain of members of the tristetraprolin protein family
Abstract
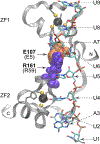
Tristetraprolin (TTP), the prototype member of the protein family of the same name, was originally discovered as the product of a rapidly inducible gene in mouse cells. Development of a knockout (KO) mouse established that absence of the protein led to a severe inflammatory syndrome, due in part to elevated levels of tumor necrosis factor (TNF). TTP was found to bind directly and with high affinity to specific AU-rich sequences in the 3'-untranslated region of the TNF mRNA. This initial binding led to promotion of TNF mRNA decay and inhibition of its translation. Many additional TTP target mRNAs have since been identified, some of which are cytokines and chemokines involved in the inflammatory response. There are three other proteins in the mouse with similar activities and domain structures, but whose KO phenotypes are remarkably different. Moreover, proteins with similar domain structures and activities have been found throughout eukaryotes, demonstrating that this protein family arose from an ancient ancestor. The defining characteristic of this protein family is the tandem zinc finger (TZF) domain, a 64 amino acid sequence with many conserved residues that is responsible for the direct RNA binding. We discuss here many aspects of this protein domain that have been elucidated since the original discovery of TTP, including its sequence conservation throughout eukarya; its apparent continued evolution in some lineages; its functional dependence on many key conserved residues; its "interchangeability" among evolutionarily distant species; and the evidence that RNA binding is required for the physiological functions of the proteins. This article is categorized under: RNA Interactions with Proteins and Other Molecules > RNA-Protein Complexes RNA Interactions with Proteins and Other Molecules > Protein-RNA Recognition RNA Interactions with Proteins and Other Molecules > Protein-RNA Interactions: Functional Implications.
Keywords: RNA binding proteins; deadenylation; mRNA decay; tristetraprolin; zinc finger proteins.
Published 2019. This article is a U.S. Government work and is in the public domain in the USA.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures





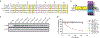

References
-
- Abbehausen C (2019). “Zinc finger domains as therapeutic targets for metal-based compounds - an update.” Metallomics 11(1): 15–28. - PubMed
-
- Blackshear PJ, Lai WS, Kennington EA, Brewer G, Wilson GM, Guan X and Zhou P (2003). “Characteristics of the interaction of a synthetic human tristetraprolin tandem zinc finger peptide with AU-rich element-containing RNA substrates.” J Biol Chem 278(22): 19947–19955. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

