Variations in study outcomes relative to intention-to-treat and per-protocol data analysis techniques in the evaluation of efficacy for treatment of venous leg ulcers with dehydrated human amnion/chorion membrane allograft
- PMID: 30864259
- PMCID: PMC6850648
- DOI: 10.1111/iwj.13094
Variations in study outcomes relative to intention-to-treat and per-protocol data analysis techniques in the evaluation of efficacy for treatment of venous leg ulcers with dehydrated human amnion/chorion membrane allograft
Abstract
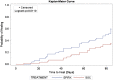
Statistical interpretation of data collected in a randomised controlled trial (RCT) is conducted on the intention-to-treat (ITT) and/or the per-protocol (PP) study populations. ITT analysis is a comparison of treatment groups including all patients as originally allocated after randomisation regardless if treatment was initiated or completed. PP analysis is a comparison of treatment groups including only those patients who completed the treatment as originally allocated, although it is often criticised because of its potential to instil bias. A previous report from an RCT conducted to evaluate the efficacy of dehydrated human amnion/chorion membrane allograft (EpiFix) as an adjunct to standard comprehensive wound therapy consisting of moist dressings and multi-layer compression in the healing of venous leg ulcers (VLUs) only reported PP study results (n = 109, 52 EpiFix and 57 standard care patients), although there were 128 patients randomised: 64 to the EpiFix group and 64 to the standard care group. Primary study outcome was the incidence of healing at 12 weeks. The purpose of the present study is to report ITT results on all 128 randomised subjects and assess if both ITT and PP data analyses arrive at the same conclusion of the efficacy of EpiFix as a treatment for VLU. Rates of healing for the ITT and PP populations were, respectively, 50% and 60% for those receiving EpiFix and 31% and 35% for those in the standard care cohort. Within both ITT and PP analyses, these differences were statistically significant; P = 0.0473, ITT and P = 0.0128, PP. The Kaplan-Meier plot of time to heal within 12 weeks for the ITT and PP populations demonstrated a superior wound-healing trajectory for EpiFix compared with VLUs treated with standard care alone. These data provide clinicians and health policymakers an additional level of assurance regarding the effectiveness of EpiFix.
Keywords: EpiFix; dehydrated human amnion/chorion membrane; intention-to-treat; venous leg ulcers.
© 2019 The Authors. International Wound Journal published by Medicalhelplines.com Inc and John Wiley & Sons Ltd.
Figures
Similar articles
-
A multicentre randomised controlled trial evaluating the efficacy of dehydrated human amnion/chorion membrane (EpiFix® ) allograft for the treatment of venous leg ulcers.Int Wound J. 2018 Feb;15(1):114-122. doi: 10.1111/iwj.12843. Epub 2017 Oct 11. Int Wound J. 2018. PMID: 29024419 Free PMC article. Clinical Trial.
-
A confirmatory study on the efficacy of dehydrated human amnion/chorion membrane dHACM allograft in the management of diabetic foot ulcers: A prospective, multicentre, randomised, controlled study of 110 patients from 14 wound clinics.Int Wound J. 2019 Feb;16(1):19-29. doi: 10.1111/iwj.12976. Epub 2018 Aug 22. Int Wound J. 2019. PMID: 30136445 Free PMC article. Clinical Trial.
-
Treatment of chronic diabetic lower extremity ulcers with advanced therapies: a prospective, randomised, controlled, multi-centre comparative study examining clinical efficacy and cost.Int Wound J. 2016 Apr;13(2):272-82. doi: 10.1111/iwj.12566. Epub 2015 Dec 23. Int Wound J. 2016. PMID: 26695998 Free PMC article. Clinical Trial.
-
Effectiveness and safety of human amnion/chorion membrane therapy for diabetic foot ulcers: An updated meta-analysis of randomized clinical trials.Wound Repair Regen. 2020 Nov;28(6):739-750. doi: 10.1111/wrr.12851. Epub 2020 Aug 11. Wound Repair Regen. 2020. PMID: 32715574
-
Amniotic Membrane Adjuncts and Clinical Applications in Wound Healing: A Review of the Literature.Wounds. 2018 Jun;30(6):168-173. Wounds. 2018. PMID: 30059334 Review.
Cited by
-
Comparison of Skin Substitutes for Acute and Chronic Wound Management.Semin Plast Surg. 2021 Aug;35(3):171-180. doi: 10.1055/s-0041-1731463. Epub 2021 Sep 10. Semin Plast Surg. 2021. PMID: 34526865 Free PMC article. Review.
-
Amnion-derived hydrogels as a versatile platform for regenerative therapy: from lab to market.Front Bioeng Biotechnol. 2024 Feb 26;12:1358977. doi: 10.3389/fbioe.2024.1358977. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 38468689 Free PMC article. Review.
-
Skin Substitutes for Adults With Diabetic Foot Ulcers and Venous Leg Ulcers: A Health Technology Assessment.Ont Health Technol Assess Ser. 2021 Jun 4;21(7):1-165. eCollection 2021. Ont Health Technol Assess Ser. 2021. PMID: 34211616 Free PMC article.
-
Assessing the Safety, Tolerability and Efficacy of Cell-Free Amniotic Fluid in the Treatment of Non-Healing Venous Ulcers: Initial Experience From a Prospective, Multicenter, Phase II Study.Int Wound J. 2025 Apr;22(4):e70171. doi: 10.1111/iwj.70171. Int Wound J. 2025. PMID: 40129130 Free PMC article. Clinical Trial.
-
LOXL2 from human amniotic mesenchymal stem cells accelerates wound epithelialization by promoting differentiation and migration of keratinocytes.Aging (Albany NY). 2020 Jul 4;12(13):12960-12986. doi: 10.18632/aging.103384. Epub 2020 Jul 4. Aging (Albany NY). 2020. PMID: 32621591 Free PMC article.
References
-
- McLafferty RB. Venous leg ulcers. In: Mowatt‐Larssen E, Desai SS, Dua A, Shortell CEK, eds. Phlebology, Vein Surgery and Ultrasonography: Diagnosis and Management of Venous Disease. Cham, Switzerland: Springer; 2014:341‐355.
-
- U.S. Food and Drug Administration . E9(R1) Statistical Principles for Clinical Trials: Addendum: Estimands and Sensitivity Analysis in Clinical Trials; 2017. https://www.federalregister.gov/documents/2017/10/31/2017‐23613/e9r1‐sta.... Accessed July 30, 2018.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous