A complex between DYRK1A and DCAF7 phosphorylates the C-terminal domain of RNA polymerase II to promote myogenesis
- PMID: 30864669
- PMCID: PMC6511856
- DOI: 10.1093/nar/gkz162
A complex between DYRK1A and DCAF7 phosphorylates the C-terminal domain of RNA polymerase II to promote myogenesis
Abstract
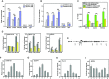
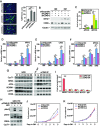
The general transcription factor P-TEFb, a master regulator of RNA polymerase (Pol) II elongation, phosphorylates the C-terminal domain (CTD) of Pol II and negative elongation factors to release Pol II from promoter-proximal pausing. We show here that P-TEFb surprisingly inhibits the myoblast differentiation into myotubes, and that P-TEFb and its two positive complexes are eliminated in this process. In contrast, DYRK1A, another CTD kinase known to control transcription of a subset of genes important for development and tissue homeostasis, is found to activate transcription of key myogenic genes. We show that active DYRK1A exists in a complex with the WD40-repeat protein DCAF7 that stabilizes and tethers DYRK1A to Pol II, so that DYRK1A-DCAF7 can co-migrate with and phosphorylate Pol II along the myogenic gene loci. Thus, DCAF7 modulates the kinase signaling output of DYRK1A on Pol II to stimulate myogenic transcription after active P-TEFb function is shut off.
© The Author(s) 2019. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures





References
-
- Phatnani H.P., Greenleaf A.L.. Phosphorylation and functions of the RNA polymerase II CTD. Genes Dev. 2006; 20:2922–2936. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

