Loss of Fam20c causes defects in the acinar and duct structure of salivary glands in mice
- PMID: 30864688
- PMCID: PMC6443332
- DOI: 10.3892/ijmm.2019.4126
Loss of Fam20c causes defects in the acinar and duct structure of salivary glands in mice
Abstract
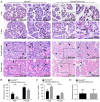
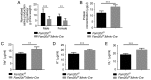
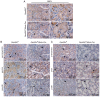
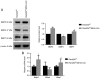
Family with sequence similarity 20‑member C (FAM20C), a recently characterized Golgi kinase, performs numerous biological functions by phosphorylating more than 100 secreted proteins. However, the role of FAM20C in the salivary glands remains undefined. The present study demonstrated that FAM20C is mainly located in the cytoplasm of duct epithelial cells in the salivary glands. Fam20cf/f; Mmtv‑Cre mice were created in which Fam20c was inactivated in the salivary gland cells and observed that the number of ducts and the ductal cross‑sectional area increased significantly, while the number of acinar cells was reduced. The granular convoluted tubules (GCTs) exhibited an accumulation of aberrant secretory granules, along with a reduced expression and altered distribution patterns of β nerve growth factor, α‑amylase and bone morphogenetic protein (BMP) 4. This abnormality suggested that the GCT cells were immature and exhibited defects in developmental and secretory functions. In accordance with the morphological alterations and the reduced number of acinar cells, FAM20C deficiency in the salivary glands significantly decreased the salivary flow rate. The Na+, Cl- and K+ concentrations in the saliva were all significantly increased due to dysfunction of the ducts. Furthermore, Fam20c deficiency significantly increased BMP2 and BMP7 expression, decreased BMP4 expression, and attenuated the canonical and noncanonical BMP signaling pathways in the salivary glands. Collectively, the results of the present study demonstrate that FAM20C is a key regulator of acinar and duct structure and duct maturation and provide a novel avenue for investigating novel therapeutic targets for oral diseases including xerostomia.
Figures






Similar articles
-
The effect and mechanism of gene Fam20a on the development and function of salivary glands in mice.Arch Oral Biol. 2022 May;137:105367. doi: 10.1016/j.archoralbio.2022.105367. Epub 2022 Feb 3. Arch Oral Biol. 2022. PMID: 35278791
-
TAT-mediated delivery of Tousled protein to salivary glands protects against radiation-induced hypofunction.Int J Radiat Oncol Biol Phys. 2012 Sep 1;84(1):257-65. doi: 10.1016/j.ijrobp.2011.10.064. Epub 2012 Jan 26. Int J Radiat Oncol Biol Phys. 2012. PMID: 22285666
-
Localization of phosphatidylinositol 4-phosphate 5-kinase (PIP5K) α, β, γ in the three major salivary glands in situ of mice and their response to β-adrenoceptor stimulation.J Anat. 2019 Apr;234(4):502-514. doi: 10.1111/joa.12944. Epub 2019 Feb 7. J Anat. 2019. PMID: 30734271 Free PMC article.
-
Calcium signaling defects underlying salivary gland dysfunction.Biochim Biophys Acta Mol Cell Res. 2018 Nov;1865(11 Pt B):1771-1777. doi: 10.1016/j.bbamcr.2018.07.002. Epub 2018 Jul 10. Biochim Biophys Acta Mol Cell Res. 2018. PMID: 30006140 Review.
-
Apoptotic cell death during regressive changes in salivary glands: A morphological perspective.J Oral Biosci. 2025 Mar;67(1):100585. doi: 10.1016/j.job.2024.100585. Epub 2024 Nov 1. J Oral Biosci. 2025. PMID: 39489340 Review.
Cited by
-
Regulation of bone morphogenetic protein 4 on epithelial tissue.J Cell Commun Signal. 2020 Sep;14(3):283-292. doi: 10.1007/s12079-019-00537-3. Epub 2020 Jan 7. J Cell Commun Signal. 2020. PMID: 31912367 Free PMC article. Review.
-
Mesenchymal-to-epithelial transition of osteoblasts induced by Fam20c knockout.Genes Genomics. 2022 Feb;44(2):155-164. doi: 10.1007/s13258-021-01170-4. Epub 2022 Jan 13. Genes Genomics. 2022. PMID: 35025083
-
Fam20c regulates the calpain proteolysis system through phosphorylating Calpasatatin to maintain cell homeostasis.J Transl Med. 2023 Jun 27;21(1):417. doi: 10.1186/s12967-023-04275-4. J Transl Med. 2023. PMID: 37370126 Free PMC article.
-
FAM20C: A key protein kinase in multiple diseases.Genes Dis. 2023 Nov 23;12(2):101179. doi: 10.1016/j.gendis.2023.101179. eCollection 2025 Mar. Genes Dis. 2023. PMID: 39790934 Free PMC article. Review.
-
From biomineralization to tumorogenesis-the expanding insight of the physiological and pathological roles of Fam20C.Biosci Rep. 2021 May 28;41(5):BSR20210040. doi: 10.1042/BSR20210040. Biosci Rep. 2021. PMID: 33942849 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

