Estrogen receptor β suppresses inflammation and the progression of prostate cancer
- PMID: 30864712
- PMCID: PMC6472045
- DOI: 10.3892/mmr.2019.10014
Estrogen receptor β suppresses inflammation and the progression of prostate cancer
Abstract
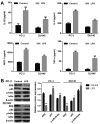
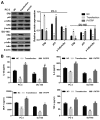
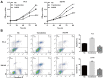
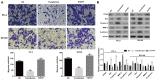
Previous studies demonstrated that estrogen receptor β (ERβ) signaling alleviates systemic inflammation in animal models, and suggested that ERβ‑selective agonists may deactivate microglia and suppress T cell activity via downregulation of nuclear factor κ‑light‑chain‑enhancer of activated B cells (NF‑κB). In the present study, the role of ERβ in lipopolysaccharide (LPS)‑induced inflammation and association with NF‑κB activity were investigated in PC‑3 and DU145 prostate cancer cell lines. Cells were treated with LPS to induce inflammation, and ELISA was performed to determine the expression levels of inflammatory cytokines, including tumor necrosis factor‑α (TNF‑α), monocyte chemoattractant protein 1 (MCP‑1), interleukin (IL)‑1β and IL‑6. MTT and Transwell assays, and Annexin V/propidium iodide staining were conducted to measure cell viability, apoptosis and migration, respectively. Protein expression was determined via western blot analysis. LPS‑induced inflammation resulted in elevated expression levels of TNF‑α, IL‑1β, MCP‑1 and IL‑6 compared with controls. ERβ overexpression significantly inhibited the LPS‑induced production of TNF‑α, IL‑1β, MCP‑1 and IL‑6. In addition, the results indicated that ERβ suppressed viability and migration, and induced apoptosis in prostate cancer cells, which was further demonstrated by altered expression of proliferating cell nuclear antigen, B‑cell lymphoma 2‑associated X protein, caspase‑3, E‑cadherin and matrix metalloproteinase‑2. These effects were reversed by treatment with the ERβ antagonist PHTPP or ERβ‑specific short interfering RNA. ERβ overexpression reduced the expression levels of p65 and phosphorylated NF‑κB inhibitor α (IκBα), but not total IκBα expression in LPS‑treated cells. In conclusion, ERβ suppressed the viability and migration of the PC‑3 and DU145 prostate cancer cell lines and induced apoptosis. Furthermore, it reduced inflammation and suppressed the activation of the NF‑κB pathway, suggesting that ERβ may serve roles as an anti‑inflammatory and anticancer agent in prostate cancer.
Figures
Similar articles
-
Signal Crosstalk and the Role of Estrogen Receptor beta (ERβ) in Prostate Cancer.Med Sci Monit. 2022 Apr 6;28:e935599. doi: 10.12659/MSM.935599. Med Sci Monit. 2022. PMID: 35383138 Free PMC article. Review.
-
Inflammation-mediated abrogation of androgen signaling: an in vitro model of prostate cell inflammation.Mol Carcinog. 2014 Feb;53(2):85-97. doi: 10.1002/mc.21948. Epub 2012 Aug 21. Mol Carcinog. 2014. PMID: 22911881
-
ERβ regulation of NF-kB activation in prostate cancer is mediated by HIF-1.Oncotarget. 2015 Nov 24;6(37):40247-54. doi: 10.18632/oncotarget.5377. Oncotarget. 2015. PMID: 26450901 Free PMC article.
-
Bakuchiol exhibits anti-metastasis activity through NF-κB cross-talk signaling with AR and ERβ in androgen-independent prostate cancer cells PC-3.J Pharmacol Sci. 2018 Sep;138(1):1-8. doi: 10.1016/j.jphs.2017.04.004. Epub 2017 Jun 16. J Pharmacol Sci. 2018. PMID: 30236540
-
The role of estrogen receptor β in prostate cancer.Mol Med. 2014 Oct 2;20(1):427-34. doi: 10.2119/molmed.2014.00105. Mol Med. 2014. PMID: 25032955 Free PMC article. Review.
Cited by
-
The Role of ERα and ERβ in Castration-Resistant Prostate Cancer and Current Therapeutic Approaches.Biomedicines. 2023 Mar 9;11(3):826. doi: 10.3390/biomedicines11030826. Biomedicines. 2023. PMID: 36979805 Free PMC article. Review.
-
Sex Differences in Colon Cancer: Genomic and Nongenomic Signalling of Oestrogen.Genes (Basel). 2023 Dec 16;14(12):2225. doi: 10.3390/genes14122225. Genes (Basel). 2023. PMID: 38137047 Free PMC article. Review.
-
Estrogen Receptor Beta (ERβ): A Ligand Activated Tumor Suppressor.Front Oncol. 2020 Oct 23;10:587386. doi: 10.3389/fonc.2020.587386. eCollection 2020. Front Oncol. 2020. PMID: 33194742 Free PMC article. Review.
-
EZH2 promotes endometriosis progression through estrogen receptor and TNFα expression.Front Endocrinol (Lausanne). 2025 Jun 24;16:1574938. doi: 10.3389/fendo.2025.1574938. eCollection 2025. Front Endocrinol (Lausanne). 2025. PMID: 40630095 Free PMC article.
-
Signal Crosstalk and the Role of Estrogen Receptor beta (ERβ) in Prostate Cancer.Med Sci Monit. 2022 Apr 6;28:e935599. doi: 10.12659/MSM.935599. Med Sci Monit. 2022. PMID: 35383138 Free PMC article. Review.
References
-
- Winchester DA, Till C, Goodman PJ, Tangen CM, Santella RM, Johnson-Pais TL, Leach RJ, Xu J, Zheng SL, Thompson IM, et al. Variation in genes involved in the immune response and prostate cancer risk in the placebo arm of the Prostate Cancer Prevention Trial. Prostate. 2015;75:1403–1418. doi: 10.1002/pros.23021. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

