Computerised cognitive training for preventing dementia in people with mild cognitive impairment
- PMID: 30864747
- PMCID: PMC6415132
- DOI: 10.1002/14651858.CD012279.pub2
Computerised cognitive training for preventing dementia in people with mild cognitive impairment
Abstract
Background: The number of people living with dementia is increasing rapidly. Clinical dementia does not develop suddenly, but rather is preceded by a period of cognitive decline beyond normal age-related change. People at this intermediate stage between normal cognitive function and clinical dementia are often described as having mild cognitive impairment (MCI). Considerable research and clinical efforts have been directed toward finding disease-modifying interventions that may prevent or delay progression from MCI to clinical dementia.
Objectives: To evaluate the effects of at least 12 weeks of computerised cognitive training (CCT) on maintaining or improving cognitive function and preventing dementia in people with mild cognitive impairment.
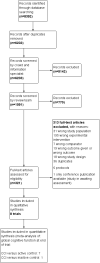
Search methods: We searched to 31 May 2018 in ALOIS (www.medicine.ox.ac.uk/alois) and ran additional searches in MEDLINE, Embase, PsycINFO, CINAHL, ClinicalTrials.gov, and the WHO portal/ICTRP (www.apps.who.int/trialsearch) to identify published, unpublished, and ongoing trials.
Selection criteria: We included randomised controlled trials (RCTs) and quasi-RCTs in which cognitive training via interactive computerised technology was compared with an active or inactive control intervention. Experimental computerised cognitive training (CCT) interventions had to adhere to the following criteria: minimum intervention duration of 12 weeks; any form of interactive computerised cognitive training, including computer exercises, computer games, mobile devices, gaming console, and virtual reality. Participants were adults with a diagnosis of mild cognitive impairment (MCI) or mild neurocognitive disorder (MND), or otherwise at high risk of cognitive decline.
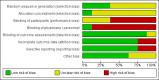
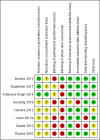
Data collection and analysis: Two review authors independently extracted data and assessed risk of bias of the included RCTs. We expressed treatment effects as mean differences (MDs) or standardised mean differences (SMDs) for continuous outcomes and as risk ratios (RRs) for dichotomous outcomes. We used the GRADE approach to describe the overall quality of evidence for each outcome.
Main results: Eight RCTs with a total of 660 participants met review inclusion criteria. Duration of the included trials varied from 12 weeks to 18 months. Only one trial used an inactive control. Most studies were at unclear or high risk of bias in several domains. Overall, our ability to draw conclusions was hampered by very low-quality evidence. Almost all results were very imprecise; there were also problems related to risk of bias, inconsistency between trials, and indirectness of the evidence.No trial provided data on incident dementia. For comparisons of CCT with both active and inactive controls, the quality of evidence on our other primary outcome of global cognitive function immediately after the intervention period was very low. Therefore, we were unable to draw any conclusions about this outcome.Due to very low quality of evidence, we were also unable to determine whether there was any effect of CCT compared to active control on our secondary outcomes of episodic memory, working memory, executive function, depression, functional performance, and mortality. We found low-quality evidence suggesting that there is probably no effect on speed of processing (SMD 0.20, 95% confidence interval (CI) -0.16 to 0.56; 2 studies; 119 participants), verbal fluency (SMD -0.16, 95% CI -0.76 to 0.44; 3 studies; 150 participants), or quality of life (mean difference (MD) 0.40, 95% CI -1.85 to 2.65; 1 study; 19 participants).When CCT was compared with inactive control, we obtained data on five secondary outcomes, including episodic memory, executive function, verbal fluency, depression, and functional performance. We found very low-quality evidence; therefore, we were unable to draw any conclusions about these outcomes.
Authors' conclusions: Currently available evidence does not allow us to determine whether or not computerised cognitive training will prevent clinical dementia or improve or maintain cognitive function in those who already have evidence of cognitive impairment. Small numbers of trials, small samples, risk of bias, inconsistency between trials, and highly imprecise results mean that it is not possible to derive any implications for clinical practice, despite some observed large effect sizes from individual studies. Direct adverse events are unlikely to occur, although the time and sometimes the money involved in computerised cognitive training programmes may represent significant burdens. Further research is necessary and should concentrate on improving methodological rigour, selecting suitable outcomes measures, and assessing generalisability and persistence of any effects. Trials with long-term follow-up are needed to determine the potential of this intervention to reduce the risk of dementia.
Conflict of interest statement
Nicola J Gates ‐ none known Robin WM Vernooij ‐ none known Marcello Di Nisio ‐ Di Nisio declares partial funding by a grant for the project 'OPERAM: OPtimising therapy to prevent Avoidable hospital admissions in the Multi‐morbid elderly' supported by the European Union's Horizon 2020 research and innovation programme under the grant agreement No 6342388. Di Nisio reports participation to Advisory Boards for Daiichi‐Sankyo, Aspen, and Pfizer, and consultancy fees for Daiichi‐Sankyo, Bayer Health Care, and Leo Pharma outside the submitted work. Salman Karim ‐ none known Evrim March ‐ none known Gabriel Martínez ‐ none known Anne WS Rutjes ‐ Dr. Rutjes declares partial funding by a grant for the project 'OPERAM: OPtimising therapy to prevent Avoidable hospital admissions in the Multi‐morbid elderly' supported by the European Union's Horizon 2020 research and innovation programme under the grant agreement No 6342388, and by the Swiss State Secretariat for Education, Research and Innovation (SERI) under contract number 15.0137.
Figures


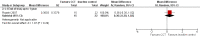















Update of
References
References to studies included in this review
-
- Djabelkhir L, Wu YH, Vidal JS, Cristancho‐Lacroix V, Marlats F, Lenoir H, et al. Computerized cognitive stimulation and engagement programs in older adults with mild cognitive impairment: comparing feasibility, acceptability, and cognitive and psychosocial effects. Clinical Interventions in Aging 2017;12:1967‐75. - PMC - PubMed
-
- Fiatarone Singh MA, Gates N, Saigal N, Wilson GC, Meiklejohn J, Brodaty H, et al. The Study of Mental and Resistance Training (SMART) study—resistance training and/or cognitive training in mild cognitive impairment: a randomized, double‐blind, double‐sham controlled trial. Journal of the American Medical Directors Association 2014;15(12):873‐80. - PubMed
-
- Gooding AL, Choi J, Fiszdon JM, Wilkins K, Kirwin PD, Dyck CH, et al. Comparing three methods of computerised cognitive training for older adults with subclinical cognitive decline. Neuropsychological Rehabilitation 2016;26(5‐6):810‐21. - PubMed
-
- Herrera C, Chambon C, Michel BF, Paban V, Alescio‐Lautier B. Positive effects of computer‐based cognitive training in adults with mild cognitive impairment. Neuropsychologia 2012;50(8):1871‐81. - PubMed
References to studies excluded from this review
-
- Adel D, Boulanouar K, Chauveau N, Delrieu J, Voisin T, Vellas B, et al. Structural MRI and FDG‐PET modifications induced by one year multidomain intervention in elderly. Conference: 26th Annual Congress of the European Association of Nuclear Medicine 2013, EANM, Lyon, France, 2013;Conference Start: 20131019 Conference End: 20131023:S208.
-
- Apóstolo JL, Cardoso DF, Rosa AI, Paúl C. The effect of cognitive stimulation on nursing home elders: a randomized controlled trial. Journal of Nursing Scholarship 2014;46(3):157‐66. - PubMed
-
- Alves J, Alves‐Costa F, Magalhães R, Gonçalves OF, Sampaio A. Cognitive stimulation for Portuguese older adults with cognitive impairment: a randomized controlled trial of efficacy, comparative duration, feasibility, and experiential relevance. American Journal of Alzheimer's Disease and Other Dementias 2014;29(6):503‐12. - PMC - PubMed
-
- Ann B, Eva E, Siv S, Elisabeth A. Effects of working memory training on functioning in daily life. Conference: 9th Annual Conference of the Special Interest Group in Neuropsychological Rehabilitation of the World Federation for NeuroRehabilitation 2012, WFNR, Bergen, Norway, 2012;Conference Start: 20120702 Conference End: 20120703:182.
Additional references
-
- Abraham RP, Denton DA, Al‐Assaf AS, Rutjes AW, Chong LY, Malik MA, et al. Vitamin and mineral supplementation for prevention of dementia or delaying cognitive decline in people with mild cognitive impairment. Cochrane Database of Systematic Reviews 2015, Issue 10. [DOI: 10.1002/14651858.CD011905] - DOI - PMC - PubMed
-
- Acevedo A, Loewenstein DA. Nonpharmacological cognitive interventions in aging and dementia. Journal of Geriatric Psychiatry and Neurology 2007;20(4):239–49. - PubMed
-
- Al‐Assaf AS, Denton DA, Abraham RP, Rutjes AW, Chong LY, Anderson JL, et al. Vitamin and mineral supplementation for maintaining cognitive function in cognitively healthy people in late life. Cochrane Database of Systematic Reviews 2015, Issue 10. [DOI: 10.1002/14651858.CD011906] - DOI - PMC - PubMed
-
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & Dementia 2011;7(3):270‐9. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

