Prompt apoptotic response to high glucose in SGLT-expressing renal cells
- PMID: 30864838
- PMCID: PMC6580252
- DOI: 10.1152/ajprenal.00615.2018
Prompt apoptotic response to high glucose in SGLT-expressing renal cells
Abstract
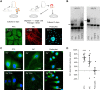
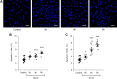
It is generally believed that cells that are unable to downregulate glucose transport are particularly vulnerable to hyperglycemia. Yet, little is known about the relation between expression of glucose transporters and acute toxic effects of high glucose exposure. In the present ex vivo study of rat renal cells, we compared the apoptotic response to a moderate increase in glucose concentration. We studied cell types that commonly are targeted in diabetic kidney disease (DKD): proximal tubule cells, which express Na+-dependent glucose transporter (SGLT)2, mesangial cells, which express SGLT1, and podocytes, which lack SGLT and take up glucose via insulin-dependent glucose transporter 4. Proximal tubule cells and mesangial cells responded within 4-8 h of exposure to 15 mM glucose with translocation of the apoptotic protein Bax to mitochondria and an increased apoptotic index. SGLT downregulation and exposure to SGLT inhibitors abolished the apoptotic response. The onset of overt DKD generally coincides with the onset of albuminuria. Albumin had an additive effect on the apoptotic response. Ouabain, which interferes with the apoptotic onset, rescued from the apoptotic response. Insulin-supplemented podocytes remained resistant to 15 and 30 mM glucose for at least 24 h. Our study points to a previously unappreciated role of SGLT-dependent glucose uptake as a risk factor for diabetic complications and highlights the importance of therapeutic approaches that specifically target the different cell types in DKD.
Keywords: apoptosis; hyperglycemia; podocytes; proximal tubular cells; sodium-dependent glucose transporter.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures






References
-
- Bonner C, Kerr-Conte J, Gmyr V, Queniat G, Moerman E, Thévenet J, Beaucamps C, Delalleau N, Popescu I, Malaisse WJ, Sener A, Deprez B, Abderrahmani A, Staels B, Pattou F. Inhibition of the glucose transporter SGLT2 with dapagliflozin in pancreatic alpha cells triggers glucagon secretion. Nat Med 21: 512–517, 2015. doi:10.1038/nm.3828. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

