Endothelial to Mesenchymal Transition: Role in Physiology and in the Pathogenesis of Human Diseases
- PMID: 30864875
- PMCID: PMC6734087
- DOI: 10.1152/physrev.00021.2018
Endothelial to Mesenchymal Transition: Role in Physiology and in the Pathogenesis of Human Diseases
Abstract
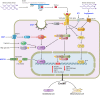
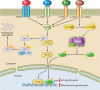
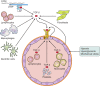
Numerous studies have demonstrated that endothelial cells are capable of undergoing endothelial to mesenchymal transition (EndMT), a newly recognized type of cellular transdifferentiation. EndMT is a complex biological process in which endothelial cells adopt a mesenchymal phenotype displaying typical mesenchymal cell morphology and functions, including the acquisition of cellular motility and contractile properties. Endothelial cells undergoing EndMT lose the expression of endothelial cell-specific proteins such as CD31/platelet-endothelial cell adhesion molecule, von Willebrand factor, and vascular-endothelial cadherin and initiate the expression of mesenchymal cell-specific genes and the production of their encoded proteins including α-smooth muscle actin, extra domain A fibronectin, N-cadherin, vimentin, fibroblast specific protein-1, also known as S100A4 protein, and fibrillar type I and type III collagens. Transforming growth factor-β1 is considered the main EndMT inducer. However, EndMT involves numerous molecular and signaling pathways that are triggered and modulated by multiple and often redundant mechanisms depending on the specific cellular context and on the physiological or pathological status of the cells. EndMT participates in highly important embryonic development processes, as well as in the pathogenesis of numerous genetically determined and acquired human diseases including malignant, vascular, inflammatory, and fibrotic disorders. Despite intensive investigation, many aspects of EndMT remain to be elucidated. The identification of molecules and regulatory pathways involved in EndMT and the discovery of specific EndMT inhibitors should provide novel therapeutic approaches for various human disorders mediated by EndMT.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures



References
-
- Abu El-Asrar AM, De Hertogh G, van den Eynde K, Alam K, Van Raemdonck K, Opdenakker G, Van Damme J, Geboes K, Struyf S. Myofibroblasts in proliferative diabetic retinopathy can originate from infiltrating fibrocytes and through endothelial-to-mesenchymal transition (EndoMT). Exp Eye Res 132: 179–189, 2015. doi:10.1016/j.exer.2015.01.023. - DOI - PubMed
-
- Agarwal S, Loder S, Cholok D, Peterson J, Li J, Fireman D, Breuler C, Hsieh HS, Ranganathan K, Hwang C, Drake J, Li S, Chan CK, Longaker MT, Levi B. Local and Circulating Endothelial Cells Undergo Endothelial to Mesenchymal Transition (EndMT) in Response to Musculoskeletal Injury. Sci Rep 6: 32514, 2016. doi:10.1038/srep32514. - DOI - PMC - PubMed
-
- Aird WC. Endothelium in health and disease. Pharmacol Rep 60: 139–143, 2008. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

