Connective auxin transport contributes to strigolactone-mediated shoot branching control independent of the transcription factor BRC1
- PMID: 30865619
- PMCID: PMC6433298
- DOI: 10.1371/journal.pgen.1008023
Connective auxin transport contributes to strigolactone-mediated shoot branching control independent of the transcription factor BRC1
Abstract
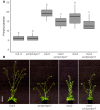
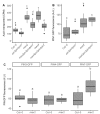
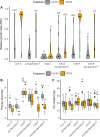
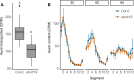
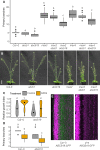
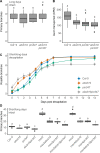
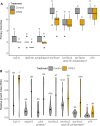
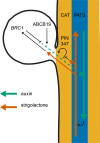
The shoot systems of plants are built by the action of the primary shoot apical meristem, established during embryogenesis. In the axil of each leaf produced by the primary meristem, secondary axillary shoot apical meristems are established. The dynamic regulation of the activity of these axillary meristems gives shoot systems their extraordinary plasticity of form. The ability of plants to activate or repress these axillary meristems appropriately requires communication between meristems that is environmentally sensitive. The transport network of the plant hormone auxin has long been implicated as a central player in this tuneable communication system, with other systemically mobile hormones, such as strigolactone and cytokinin, acting in part by modulating auxin transport. Until recently, the polar auxin transport stream, which provides a high conductance auxin transport route down stems dominated by the auxin export protein PIN-FORMED1 (PIN1), has been the focus for understanding long range auxin transport in the shoot. However, recently additional auxin exporters with important roles in the shoot have been identified, including PIN3, PIN4 and PIN7. These proteins contribute to a wider less polar stem auxin transport regime, which we have termed connective auxin transport (CAT), because of its role in communication across the shoot system. Here we present a genetic analysis of the role of CAT in shoot branching. We demonstrate that in Arabidopsis, CAT plays an important role in strigolactone-mediated shoot branching control, with the triple pin3pin4pin7 mutant able to suppress partially the highly branched phenotype of strigolactone deficient mutants. In contrast, the branchy phenotype of mutants lacking the axillary meristem-expressed transcription factor, BRANCHED1 (BRC1) is unaffected by pin3pin4pin7. We further demonstrate that mutation in the ABCB19 auxin export protein, which like PIN3 PIN4 and PIN7 is widely expressed in stems, has very different effects, implicating ABCB19 in auxin loading at axillary bud apices.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Strigolactone can promote or inhibit shoot branching by triggering rapid depletion of the auxin efflux protein PIN1 from the plasma membrane.PLoS Biol. 2013;11(1):e1001474. doi: 10.1371/journal.pbio.1001474. Epub 2013 Jan 29. PLoS Biol. 2013. PMID: 23382651 Free PMC article.
-
Cytokinin Targets Auxin Transport to Promote Shoot Branching.Plant Physiol. 2018 Jun;177(2):803-818. doi: 10.1104/pp.17.01691. Epub 2018 May 1. Plant Physiol. 2018. PMID: 29717021 Free PMC article.
-
The Arabidopsis MAX pathway controls shoot branching by regulating auxin transport.Curr Biol. 2006 Mar 21;16(6):553-63. doi: 10.1016/j.cub.2006.01.058. Curr Biol. 2006. PMID: 16546078
-
Auxin and strigolactones in shoot branching: intimately connected?Biochem Soc Trans. 2010 Apr;38(2):717-22. doi: 10.1042/BST0380717. Biochem Soc Trans. 2010. PMID: 20298249 Review.
-
The fall and rise of apical dominance.Curr Opin Genet Dev. 2005 Aug;15(4):468-71. doi: 10.1016/j.gde.2005.06.010. Curr Opin Genet Dev. 2005. PMID: 15964756 Review.
Cited by
-
Overexpression of GmMYB14 improves high-density yield and drought tolerance of soybean through regulating plant architecture mediated by the brassinosteroid pathway.Plant Biotechnol J. 2021 Apr;19(4):702-716. doi: 10.1111/pbi.13496. Epub 2020 Nov 23. Plant Biotechnol J. 2021. PMID: 33098207 Free PMC article.
-
Elucidating connections between the strigolactone biosynthesis pathway, flavonoid production and root system architecture in Arabidopsis thaliana.Physiol Plant. 2022 Mar;174(2):e13681. doi: 10.1111/ppl.13681. Physiol Plant. 2022. PMID: 35362177 Free PMC article.
-
Lessons from a century of apical dominance research.J Exp Bot. 2023 Aug 3;74(14):3903-3922. doi: 10.1093/jxb/erad137. J Exp Bot. 2023. PMID: 37076257 Free PMC article.
-
Auxin Interactions with Other Hormones in Plant Development.Cold Spring Harb Perspect Biol. 2021 Oct 1;13(10):a039990. doi: 10.1101/cshperspect.a039990. Cold Spring Harb Perspect Biol. 2021. PMID: 33903155 Free PMC article. Review.
-
Effects of light spectrum on the morphophysiology and gene expression of lateral branching in Pepino (Solanum muricatum).Front Plant Sci. 2022 Sep 23;13:1012086. doi: 10.3389/fpls.2022.1012086. eCollection 2022. Front Plant Sci. 2022. PMID: 36212344 Free PMC article.
References
-
- Ljung K, Bhalerao RP, Sandberg G. Sites and homeostatic control of auxin biosynthesis in Arabidopsis during vegetative growth. Plant J. 2001;28(4):465–74. - PubMed
-
- Goldsmith MHM. The Polar Transport of Auxin. Annu Rev Plant Biol. 1977;28(1):439–78.
-
- Gälweiler L, Guan C, Müller A, Wisman E, Mendgen K, Yephremov A, et al. Regulation of Polar Auxin Transport by AtPIN1 in Arabidopsis Vascular Tissue. Science. 1998;282(5397):2226–30. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

