Environment-induced same-sex mating in the yeast Candida albicans through the Hsf1-Hsp90 pathway
- PMID: 30865631
- PMCID: PMC6415874
- DOI: 10.1371/journal.pbio.2006966
Environment-induced same-sex mating in the yeast Candida albicans through the Hsf1-Hsp90 pathway
Abstract
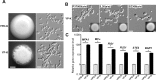
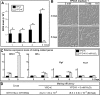
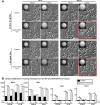
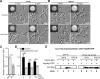
While sexual reproduction is pervasive in eukaryotic cells, the strategies employed by fungal species to achieve and complete sexual cycles is highly diverse and complex. Many fungi, including Saccharomyces cerevisiae and Schizosaccharomyces pombe, are homothallic (able to mate with their own mitotic descendants) because of homothallic switching (HO) endonuclease-mediated mating-type switching. Under laboratory conditions, the human fungal pathogen Candida albicans can undergo both heterothallic and homothallic (opposite- and same-sex) mating. However, both mating modes require the presence of cells with two opposite mating types (MTLa/a and α/α) in close proximity. Given the predominant clonal feature of this yeast in the human host, both opposite- and same-sex mating would be rare in nature. In this study, we report that glucose starvation and oxidative stress, common environmental stresses encountered by the pathogen, induce the development of mating projections and efficiently permit same-sex mating in C. albicans with an "a" mating type (MTLa/a). This induction bypasses the requirement for the presence of cells with an opposite mating type and allows efficient sexual mating between cells derived from a single progenitor. Glucose starvation causes an increase in intracellular oxidative species, overwhelming the Heat Shock transcription Factor 1 (Hsf1)- and Heat shock protein (Hsp)90-mediated stress-response pathway. We further demonstrate that Candida TransActivating protein 4 (Cta4) and Cell Wall Transcription factor 1 (Cwt1), downstream effectors of the Hsf1-Hsp90 pathway, regulate same-sex mating in C. albicans through the transcriptional control of the master regulator of a-type mating, MTLa2, and the pheromone precursor-encoding gene Mating α factor precursor (MFα). Our results suggest that mating could occur much more frequently in nature than was originally appreciated and that same-sex mating could be an important mode of sexual reproduction in C. albicans.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Hull CM, Raisner RM, Johnson AD. Evidence for mating of the "asexual" yeast Candida albicans in a mammalian host. Science. 2000;289(5477):307–10. . - PubMed
-
- Magee BB, Magee PT. Induction of mating in Candida albicans by construction of MTLa and MTLalpha strains. Science. 2000;289(5477):310–3. Epub 2000/07/15. 8662 [pii]. . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

