Seasonal malaria chemoprevention combined with community case management of malaria in children under 10 years of age, over 5 months, in south-east Senegal: A cluster-randomised trial
- PMID: 30865632
- PMCID: PMC6415816
- DOI: 10.1371/journal.pmed.1002762
Seasonal malaria chemoprevention combined with community case management of malaria in children under 10 years of age, over 5 months, in south-east Senegal: A cluster-randomised trial
Abstract
Background: Seasonal malaria chemoprevention (SMC) is recommended in the Sahel region of Africa for children under 5 years of age, for up to 4 months of the year. It may be appropriate to include older children, and to provide protection for more than 4 months. We evaluated the effectiveness of SMC using sulfadoxine-pyrimethamine plus amodiaquine given over 5 months to children under 10 years of age in Saraya district in south-east Senegal in 2011.
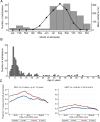
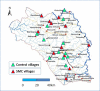
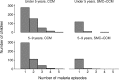
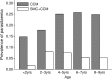
Methods and findings: Twenty-four villages, including 2,301 children aged 3-59 months and 2,245 aged 5-9 years, were randomised to receive SMC with community case management (CCM) (SMC villages) or CCM alone (control villages). In all villages, community health workers (CHWs) were trained to treat malaria cases with artemisinin combination therapy after testing with a rapid diagnostic test (RDT). In SMC villages, CHWs administered SMC to children aged 3 months to 9 years once a month for 5 months. The study was conducted from 27 July to 31 December 2011. The primary outcome was malaria (fever or history of fever with a positive RDT). The prevalence of anaemia and parasitaemia was measured in a survey at the end of the transmission season. Molecular markers associated with resistance to SMC drugs were analysed in samples from incident malaria cases and from children with parasitaemia in the survey. SMC was well tolerated with no serious adverse reactions. There were 1,472 RDT-confirmed malaria cases in the control villages and 270 in the SMC villages. Among children under 5 years of age, the rate difference was 110.8/1,000/month (95% CI 64.7, 156.8; p < 0.001) and among children 5-9 years of age, 101.3/1,000/month (95% CI 66.7, 136.0; p < 0.001). The mean haemoglobin concentration at the end of the transmission season was higher in SMC than control villages, by 6.5 g/l (95% CI 2.0, 11; p = 0.007) among children under 5 years of age, and by 5.2 g/l (95% CI 0.4, 9.9; p = 0.035) among children 5-9 years of age. The prevalence of parasitaemia was 18% in children under 5 years of age and 25% in children 5-9 years of age in the control villages, and 5.7% and 5.8%, respectively, in these 2 age groups in the SMC villages, with prevalence differences of 12.5% (95% CI 6.8%, 18.2%; p < 0.001) in children under 5 years of age and 19.3% (95% CI 8.3%, 30.2%; p < 0.001) in children 5-9 years of age. The pfdhps-540E mutation associated with clinical resistance to sulfadoxine-pyrimethamine was found in 0.8% of samples from malaria cases but not in the final survey. Twelve children died in the control group and 14 in the SMC group, a rate difference of 0.096/1,000 child-months (95% CI 0.99, 1.18; p = 0.895). Limitations of this study include that we were not able to obtain blood smears for microscopy for all suspected malaria cases, such that we had to rely on RDTs for confirmation, which may have included false positives.
Conclusions: In this study SMC for children under 10 years of age given over 5 months was feasible, well tolerated, and effective in preventing malaria episodes, and reduced the prevalence of parasitaemia and anaemia. SMC with CCM achieved high coverage and ensured children with malaria were promptly treated with artemether-lumefantrine.
Trial registration: www.clinicaltrials.gov NCT01449045.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- World Health Organization. Seasonal malaria chemoprevention with sulfadoxine-pyrimethamine plus amodiaquine in children: a field guide. Geneva: World Health Organization; 2013.
-
- Ndiaye JL, Cissé B, Ba EH, Gomis JF, Ndour CT, Molez JF, et al. Safety of seasonal malaria chemoprevention (SMC) with sulfadoxine-pyrimethamine plus amodiaquine when delivered to children under 10 years of age by district health services in Senegal: results from a stepped wedge cluster randomized trial. PLoS ONE. 2016;11(12):e0168421 10.1371/journal.pone.0168421 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

