Pasakbumin A controls the growth of Mycobacterium tuberculosis by enhancing the autophagy and production of antibacterial mediators in mouse macrophages
- PMID: 30865638
- PMCID: PMC6415846
- DOI: 10.1371/journal.pone.0199799
Pasakbumin A controls the growth of Mycobacterium tuberculosis by enhancing the autophagy and production of antibacterial mediators in mouse macrophages
Abstract
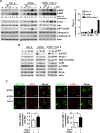
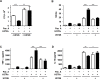
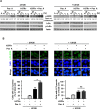
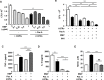
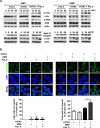
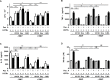
Tuberculosis (TB) is a chronic infectious disease caused by Mycobacterium tuberculosis (Mtb) and remains a major health problem worldwide. Thus, identification of new and more effective drugs to treat emerging multidrug-resistant TB (MDR-TB) and to reduce the side effects of anti-TB drugs, such as liver toxicity and other detrimental changes, is urgently needed. In this study, to develop a novel candidate drug for effective TB treatment with few side effects in the host, we selected pasakbumin A isolated from Eurycoma longifolia (E. longifolia) Jack, which protected host cells against Mtb infection-induced death. Pasakbumin A significantly inhibited intracellular Mtb growth by inducing the autophagy via the ERK1/2-mediated signaling pathway in Mtb-infected macrophages. We further investigated whether pasakbumin A could be used as a potential adjuvant for TB treatment. Treatment with pasakbumin A and anti-TB drug rifampicin (RMP) potently suppressed intracellular Mtb killing by promoting autophagy as well as TNF-α production via the ERK1/2- and NF-κB-mediated signaling pathways in Mtb-infected cells. Our results suggest that pasakbumin A could be developed as a novel anti-TB drug or host-directed therapeutic (HDT) strategy to protect against host cell death and improve host defense mechanisms against Mtb infection in macrophages.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

