NAP (davunetide) preferential interaction with dynamic 3-repeat Tau explains differential protection in selected tauopathies
- PMID: 30865715
- PMCID: PMC6415897
- DOI: 10.1371/journal.pone.0213666
NAP (davunetide) preferential interaction with dynamic 3-repeat Tau explains differential protection in selected tauopathies
Abstract
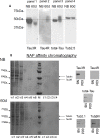
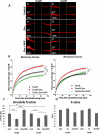
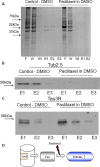
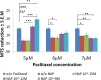
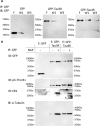
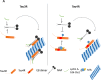
The microtubule (MT) associated protein Tau is instrumental for the regulation of MT assembly and dynamic instability, orchestrating MT-dependent cellular processes. Aberration in Tau post-translational modifications ratio deviation of spliced Tau isoforms 3 or 4 MT binding repeats (3R/4R) have been implicated in neurodegenerative tauopathies. Activity-dependent neuroprotective protein (ADNP) is vital for brain formation and cognitive function. ADNP deficiency in mice causes pathological Tau hyperphosphorylation and aggregation, correlated with impaired cognitive functions. It has been previously shown that the ADNP-derived peptide NAP protects against ADNP deficiency, exhibiting neuroprotection, MT interaction and memory protection. NAP prevents MT degradation by recruitment of Tau and end-binding proteins to MTs and expression of these proteins is required for NAP activity. Clinically, NAP (davunetide, CP201) exhibited efficacy in prodromal Alzheimer's disease patients (Tau3R/4R tauopathy) but not in progressive supranuclear palsy (increased Tau4R tauopathy). Here, we examined the potential preferential interaction of NAP with 3R vs. 4R Tau, toward personalized treatment of tauopathies. Affinity-chromatography showed that NAP preferentially interacted with Tau3R protein from rat brain extracts and fluorescence recovery after photobleaching assay indicated that NAP induced increased recruitment of human Tau3R to MTs under zinc intoxication, in comparison to Tau4R. Furthermore, we showed that NAP interaction with tubulin (MTs) was inhibited by obstruction of Tau-binding sites on MTs, confirming the requirement of Tau-MT interaction for NAP activity. The preferential interaction of NAP with Tau3R may explain clinical efficacy in mixed vs. Tau4R pathologies, and suggest effectiveness in Tau3R neurodevelopmental disorders.
Conflict of interest statement
Professor Gozes also serves as the Chief Scientific Officer of Coronis Neurosciences, developing CP201 for the ADNP syndrome, under patent protection. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures

References
-
- Lee VM, Balin BJ, Otvos L Jr., Trojanowski JQ. A68: a major subunit of paired helical filaments and derivatized forms of normal Tau. Science. 1991;251(4994):675–8. . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

