One Month of Rifapentine plus Isoniazid to Prevent HIV-Related Tuberculosis
- PMID: 30865794
- PMCID: PMC6563914
- DOI: 10.1056/NEJMoa1806808
One Month of Rifapentine plus Isoniazid to Prevent HIV-Related Tuberculosis
Abstract
Background: Tuberculosis is the leading killer of patients with human immunodeficiency virus (HIV) infection. Preventive therapy is effective, but current regimens are limited by poor implementation and low completion rates.
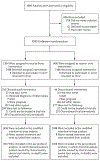
Methods: We conducted a randomized, open-label, phase 3 noninferiority trial comparing the efficacy and safety of a 1-month regimen of daily rifapentine plus isoniazid (1-month group) with 9 months of isoniazid alone (9-month group) in HIV-infected patients who were living in areas of high tuberculosis prevalence or who had evidence of latent tuberculosis infection. The primary end point was the first diagnosis of tuberculosis or death from tuberculosis or an unknown cause. Noninferiority would be shown if the upper limit of the 95% confidence interval for the between-group difference in the number of events per 100 person-years was less than 1.25.
Results: A total of 3000 patients were enrolled and followed for a median of 3.3 years. Of these patients, 54% were women; the median CD4+ count was 470 cells per cubic millimeter, and half the patients were receiving antiretroviral therapy. The primary end point was reported in 32 of 1488 patients (2%) in the 1-month group and in 33 of 1498 (2%) in the 9-month group, for an incidence rate of 0.65 per 100 person-years and 0.67 per 100 person-years, respectively (rate difference in the 1-month group, -0.02 per 100 person-years; upper limit of the 95% confidence interval, 0.30). Serious adverse events occurred in 6% of the patients in the 1-month group and in 7% of those in the 9-month group (P = 0.07). The percentage of treatment completion was significantly higher in the 1-month group than in the 9-month group (97% vs. 90%, P<0.001).
Conclusions: A 1-month regimen of rifapentine plus isoniazid was noninferior to 9 months of isoniazid alone for preventing tuberculosis in HIV-infected patients. The percentage of patients who completed treatment was significantly higher in the 1-month group. (Funded by the National Institute of Allergy and Infectious Diseases; BRIEF TB/A5279 ClinicalTrials.gov number, NCT01404312.).
Copyright © 2019 Massachusetts Medical Society.
Figures

Comment in
-
Ending Tuberculosis through Prevention.N Engl J Med. 2019 Mar 14;380(11):1073-1074. doi: 10.1056/NEJMe1901656. N Engl J Med. 2019. PMID: 30865803 No abstract available.
-
One Month of Rifapentine plus Isoniazid to Prevent HIV-Related Tuberculosis.N Engl J Med. 2019 Sep 12;381(11):e23. doi: 10.1056/NEJMc1908492. N Engl J Med. 2019. PMID: 31509689 No abstract available.
References
-
- Global tuberculosis report 2018. Geneva: World Health Organization, 2018. (https://www.who.int/tb/publications/global_report/en/).
-
- The TEMPRANO ANRS 12136 Study Group. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med 2015; 373: 808–22. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- UM1 AI069423/AI/NIAID NIH HHS/United States
- UM1 AI069456/AI/NIAID NIH HHS/United States
- P30 AI094189/AI/NIAID NIH HHS/United States
- U01 AI069463/AI/NIAID NIH HHS/United States
- UM1 AI068634/AI/NIAID NIH HHS/United States
- UM1 AI069481/AI/NIAID NIH HHS/United States
- UM1 AI069399/AI/NIAID NIH HHS/United States
- D43 TW010062/TW/FIC NIH HHS/United States
- UM1 AI069463/AI/NIAID NIH HHS/United States
- UM1 AI106701/AI/NIAID NIH HHS/United States
- UM1 AI108568/AI/NIAID NIH HHS/United States
- UM1 AI069436/AI/NIAID NIH HHS/United States
- U01 AI069436/AI/NIAID NIH HHS/United States
- HHSN-275201300003C/NH/NIH HHS/United States
- UM1 AI068636/AI/NIAID NIH HHS/United States
- UM1 AI069418/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
