Key Role of Reactive Oxygen Species (ROS) in Indirubin Derivative-Induced Cell Death in Cutaneous T-Cell Lymphoma Cells
- PMID: 30866411
- PMCID: PMC6429192
- DOI: 10.3390/ijms20051158
Key Role of Reactive Oxygen Species (ROS) in Indirubin Derivative-Induced Cell Death in Cutaneous T-Cell Lymphoma Cells
Abstract
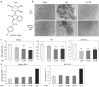
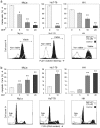
Cutaneous T-cell lymphoma (CTCL) may develop a highly malignant phenotype in its late phase, and patients may profit from innovative therapies. The plant extract indirubin and its chemical derivatives represent new and promising antitumor strategies. This first report on the effects of an indirubin derivative in CTCL cells shows a strong decrease of cell proliferation and cell viability as well as an induction of apoptosis, suggesting indirubin derivatives for therapy of CTCL. As concerning the mode of activity, the indirubin derivative DKP-071 activated the extrinsic apoptosis cascade via caspase-8 and caspase-3 through downregulation of the caspase antagonistic proteins c-FLIP and XIAP. Importantly, a strong increase of reactive oxygen species (ROS) was observed as an immediate early effect in response to DKP-071 treatment. The use of antioxidative pre-treatment proved the decisive role of ROS, which turned out upstream of all other proapoptotic effects monitored. Thus, reactive oxygen species appear as a highly active proapoptotic pathway in CTCL, which may be promising for therapeutic intervention. This pathway can be efficiently activated by an indirubin derivative.
Keywords: CTCL; XIAP; apoptosis; c-FLIP; cell viability.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Targeting Cutaneous T-Cell Lymphoma Cells by Ingenol Mebutate (PEP005) Correlates with PKCδ Activation, ROS Induction as Well as Downregulation of XIAP and c-FLIP.Cells. 2021 Apr 23;10(5):987. doi: 10.3390/cells10050987. Cells. 2021. PMID: 33922439 Free PMC article.
-
Crucial role of reactive oxygen species (ROS) for the proapoptotic effects of indirubin derivative DKP-073 in melanoma cells.Mol Carcinog. 2019 Feb;58(2):258-269. doi: 10.1002/mc.22924. Epub 2018 Nov 2. Mol Carcinog. 2019. PMID: 30320471
-
Analysis of the Effect of Gentian Violet on Apoptosis and Proliferation in Cutaneous T-Cell Lymphoma in an In Vitro Study.JAMA Dermatol. 2018 Oct 1;154(10):1191-1198. doi: 10.1001/jamadermatol.2018.2756. JAMA Dermatol. 2018. PMID: 30167641 Free PMC article.
-
Cutaneous T cell lymphoma: the helping hand of dendritic cells.Ann N Y Acad Sci. 2001 Sep;941:1-11. Ann N Y Acad Sci. 2001. PMID: 11594563 Review.
-
New drugs in cutaneous T-cell lymphomas.Curr Opin Oncol. 2016 Sep;28(5):384-9. doi: 10.1097/CCO.0000000000000311. Curr Opin Oncol. 2016. PMID: 27390044 Review.
Cited by
-
N-Glycosides of indigo, indirubin, and isoindigo: blue, red, and yellow sugars and their cancerostatic activity.Beilstein J Org Chem. 2024 Nov 8;20:2840-2869. doi: 10.3762/bjoc.20.240. eCollection 2024. Beilstein J Org Chem. 2024. PMID: 39530076 Free PMC article. Review.
-
A Thia-Analogous Indirubin N-Glycoside Disrupts Mitochondrial Function and Causes the Death of Human Melanoma and Cutaneous Squamous Cell Carcinoma Cells.Cells. 2023 Oct 5;12(19):2409. doi: 10.3390/cells12192409. Cells. 2023. PMID: 37830623 Free PMC article.
-
Inhibition of Cell Proliferation and Cell Viability by Sinecatechins in Cutaneous SCC Cells Is Related to an Imbalance of ROS and Loss of Mitochondrial Membrane Potential.Antioxidants (Basel). 2022 Jul 21;11(7):1416. doi: 10.3390/antiox11071416. Antioxidants (Basel). 2022. PMID: 35883905 Free PMC article.
-
Countering TRAIL Resistance in Melanoma.Cancers (Basel). 2019 May 11;11(5):656. doi: 10.3390/cancers11050656. Cancers (Basel). 2019. PMID: 31083589 Free PMC article. Review.
-
A Sensitive LC-MS/MS Method for the Simultaneous Determination of Two Thia-Analogous Indirubin N-Glycosides and Indirubin-3'-Monoxime in Plasma and Cell Culture Medium.Molecules. 2022 May 9;27(9):3031. doi: 10.3390/molecules27093031. Molecules. 2022. PMID: 35566381 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

