Dual-Specificity Phosphatases in Neuroblastoma Cell Growth and Differentiation
- PMID: 30866462
- PMCID: PMC6429076
- DOI: 10.3390/ijms20051170
Dual-Specificity Phosphatases in Neuroblastoma Cell Growth and Differentiation
Abstract
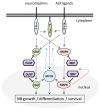
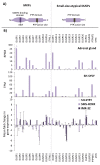
Dual-specificity phosphatases (DUSPs) are important regulators of neuronal cell growth and differentiation by targeting proteins essential to neuronal survival in signaling pathways, among which the MAP kinases (MAPKs) stand out. DUSPs include the MAPK phosphatases (MKPs), a family of enzymes that directly dephosphorylate MAPKs, as well as the small-size atypical DUSPs, a group of low molecular-weight enzymes which display more heterogeneous substrate specificity. Neuroblastoma (NB) is a malignancy intimately associated with the course of neuronal and neuroendocrine cell differentiation, and constitutes the source of more common extracranial solid pediatric tumors. Here, we review the current knowledge on the involvement of MKPs and small-size atypical DUSPs in NB cell growth and differentiation, and discuss the potential of DUSPs as predictive biomarkers and therapeutic targets in human NB.
Keywords: MAP kinase phosphatases; MAP kinases; atypical dual-specificity phosphatases; dual-specificity phosphatases; neuroblastoma; neuronal differentiation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

