Expanding the Role of the Histone Lysine-Specific Demethylase LSD1 in Cancer
- PMID: 30866496
- PMCID: PMC6468368
- DOI: 10.3390/cancers11030324
Expanding the Role of the Histone Lysine-Specific Demethylase LSD1 in Cancer
Abstract
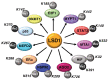
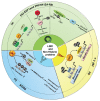
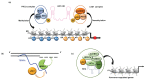
Studies of alterations in histone methylation in cancer have led to the identification of histone methyltransferases and demethylases as novel targets for therapy. Lysine-specific demethylase 1 (LSD1, also known as KDM1A), demethylates H3K4me1/2, or H3K9me1/2 in a context-dependent manner. In addition to the well-studied role of LSD1 in the epigenetic regulation of histone methylation changes, LSD1 regulates the methylation dynamic of several non-histone proteins and participates in the assembly of different long noncoding RNA (lncRNA_ complexes. LSD1 is highly expressed in various cancers, playing a pivotal role in different cancer-related processes. Here, we summarized recent findings on the role of LSD1 in the regulation of different biological processes in cancer cells through dynamic methylation of non-histone proteins and physical association with dedicated lncRNA.
Keywords: LSD1; cancer; epigenetics; histone demethylase; lncRNAs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
A comprehensive review of lysine-specific demethylase 1 and its roles in cancer.Epigenomics. 2017 Aug;9(8):1123-1142. doi: 10.2217/epi-2017-0022. Epub 2017 Jul 12. Epigenomics. 2017. PMID: 28699367 Review.
-
LSD1: Expanding Functions in Stem Cells and Differentiation.Cells. 2021 Nov 20;10(11):3252. doi: 10.3390/cells10113252. Cells. 2021. PMID: 34831474 Free PMC article. Review.
-
LSD1 demethylates histone and non-histone proteins.Epigenetics. 2009 Apr 1;4(3):129-32. doi: 10.4161/epi.4.3.8443. Epub 2009 Apr 14. Epigenetics. 2009. PMID: 19395867
-
Histone methyl-transferases and demethylases in the autophagy regulatory network: the emerging role of KDM1A/LSD1 demethylase.Autophagy. 2019 Feb;15(2):187-196. doi: 10.1080/15548627.2018.1520546. Epub 2018 Sep 22. Autophagy. 2019. PMID: 30208749 Free PMC article. Review.
-
Expression, Purification, and Biochemical Analysis of the LSD1/KDM1A Histone Demethylase.Methods Enzymol. 2016;573:241-59. doi: 10.1016/bs.mie.2016.02.001. Epub 2016 Mar 4. Methods Enzymol. 2016. PMID: 27372756
Cited by
-
LSD1-mediated demethylation of OCT4 safeguards pluripotent stem cells by maintaining the transcription of PORE-motif-containing genes.Sci Rep. 2021 May 13;11(1):10285. doi: 10.1038/s41598-021-89734-y. Sci Rep. 2021. PMID: 33986438 Free PMC article.
-
Targeting lysine-specific demethylase 1 (KDM1A/LSD1) impairs colorectal cancer tumorigenesis by affecting cancer cells stemness, motility, and differentiation.Cell Death Discov. 2023 Jun 29;9(1):201. doi: 10.1038/s41420-023-01502-1. Cell Death Discov. 2023. PMID: 37385999 Free PMC article.
-
Epigenetics in Breast Cancer Therapy-New Strategies and Future Nanomedicine Perspectives.Cancers (Basel). 2020 Dec 3;12(12):3622. doi: 10.3390/cancers12123622. Cancers (Basel). 2020. PMID: 33287297 Free PMC article. Review.
-
LSD1 regulates the FOXF2-mediated Wnt/β-catenin signaling pathway by interacting with Ku80 to promote colon cancer progression.Am J Cancer Res. 2022 Aug 15;12(8):3693-3712. eCollection 2022. Am J Cancer Res. 2022. PMID: 36119820 Free PMC article.
-
Glucose Activates Lysine-Specific Demethylase 1 through the KEAP1/p62 Pathway.Antioxidants (Basel). 2021 Nov 26;10(12):1898. doi: 10.3390/antiox10121898. Antioxidants (Basel). 2021. PMID: 34942999 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

