CAFs and TGF-β Signaling Activation by Mast Cells Contribute to Resistance to Gemcitabine/Nabpaclitaxel in Pancreatic Cancer
- PMID: 30866547
- PMCID: PMC6468868
- DOI: 10.3390/cancers11030330
CAFs and TGF-β Signaling Activation by Mast Cells Contribute to Resistance to Gemcitabine/Nabpaclitaxel in Pancreatic Cancer
Abstract
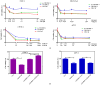
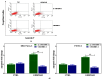
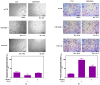
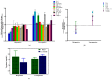
Tumor⁻stroma interactions are of key importance for pancreatic ductal adenocarcinoma (PDAC) progression. Our aim was to investigate whether cancer associated fibroblasts (CAFs) and mast cells (MC) affected the sensitivity of PDAC cells to gemcitabine/nabpaclitaxel (GEM/NAB). For this purpose, the combination cytotoxicity and the effect on tumor invasion and angiogenesis were evaluated with or without a conditioned medium from the mast cell line HMC-1 (human mast cell line-1 cells) and CAFs. Beside the clinical outcome of a homogenous population of PDAC patients, receiving GEM/NAB, was correlated to the circulating levels of mast cell tryptase and to a panel of inflammatory and immunosuppressive cytokines. CAFs neither affected drugs' cytotoxicity nor the inhibition of angiogenesis, but promoted tumor cell invasion. The MC instead, caused resistance to drugs by reducing apoptosis, by activating the TGF-β signalling and by promoting tumor invasion. Indeed, the inhibition of TβRI serine/threonine kinase activity by galunisertib restored drugs cytotoxicity. Moreover, MC induced the release of TGF-β1, and increased expression of PAR-2, ERK1/2 and Akt activation. Accordingly, TGF-β1, tryptase and other pro-inflammatory and immunosuppressive cytokines increased in the unresponsive patients. In conclusion, MC play a pivotal role in the resistance to GEM/NAB. A correlation between high level of circulating pro-inflammatory/ immunosuppressive cytokines and unresponsiveness was found in PDAC patients.
Keywords: CAFs; gemcitabine; mast cells; nabpaclitaxel; pancreatic cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Goldstein D., El-Maraghi R.H., Hammel P., Heinemann V., Kunzmann V., Sastre J., Scheithauer W., Siena S., Tabernero J., Teixeira L., et al. nab-Paclitaxel Plus Gemcitabine for Metastatic Pancreatic Cancer: Long-Term Survival From a Phase III Trial. JNCI J. Natl. Cancer Inst. 2015;107:dju413. doi: 10.1093/jnci/dju413. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

