Analysis of the Effectiveness of Lornoxicam and Flurbiprofen on Management of Pain and Sequelae Following Third Molar Surgery: A Randomized, Controlled, Clinical Trial
- PMID: 30866586
- PMCID: PMC6463050
- DOI: 10.3390/jcm8030325
Analysis of the Effectiveness of Lornoxicam and Flurbiprofen on Management of Pain and Sequelae Following Third Molar Surgery: A Randomized, Controlled, Clinical Trial
Abstract
The aim of this study was to analyze the effectiveness of Lornoxicam and Flurbiprofen in reducing perioperative sequelae after impacted mandibular third molar surgery. Ninety-one patients who needed surgical extraction of an impacted mandibular third molar were selected for the study. All subjects were randomly allocated to receive one of the following treatments twice a day for 5 days after surgery: placebo (n = 29), Flurbiprofen (n = 31), or Lornoxicam (n = 31). The primary outcome was postoperative pain, evaluated using the visual analogue scale (VAS) score at 30 min, 2, 6, 12, 24, 48 h, 7 and 10 days following surgery. The secondary outcomes chosen were changes in postoperative swelling and maximum mouth opening values compared to preoperative ones. Compared to placebo, treatment with Flurbiprofen and Lornoxicam was characterised by an improvement in the primary outcome. Moreover, the treatment with Lornoxicam presented significantly lower median pain scores at 2 h (p < 0.001) and at 6 h (p = 0.016) compared to Flurbiprofen and at 2 h (p < 0.001), 6 h (p = 0.01), and at 24 h (p = 0.018) after surgery compared with placebo. Swelling and maximum mouth opening values were not significantly different between the groups at each follow-up session. This trial demonstrated that treatment with Lornoxicam showed a decrease in the incidence and severity of pain in the first postoperative phase following third molar surgery compared to Flurbiprofen and placebo.
Keywords: Flurbiprofen; Lornoxicam; pain; randomized clinical trial; swelling; third molar surgery; trismus.
Conflict of interest statement
The authors declare that they have no conflicts of interest in the present study.
Figures
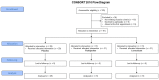

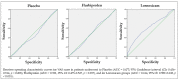
References
-
- Isola G., Matarese M., Ramaglia L., Iorio-Siciliano V., Cordasco G., Matarese G. Efficacy of a drug composed of herbal extracts on postoperative discomfort after surgical removal of impacted mandibular third molar: A randomized, triple-blind, controlled clinical trial. Clin. Oral Investig. 2018:1–11. doi: 10.1007/s00784-018-2690-9. - DOI - PubMed
LinkOut - more resources
Full Text Sources

