Efficacy and safety of Ex-PRESS® mini shunt surgery versus trabeculectomy for neovascular glaucoma: a retrospective comparative study
- PMID: 30866871
- PMCID: PMC6416865
- DOI: 10.1186/s12886-019-1083-4
Efficacy and safety of Ex-PRESS® mini shunt surgery versus trabeculectomy for neovascular glaucoma: a retrospective comparative study
Abstract
Background: The objective of this study is to evaluate and compare the short-term efficacy and safety of Ex-PRESS® mini shunt surgery and trabeculectomy for neovascular glaucoma (NVG).
Methods: Patients with NVG who underwent Ex-PRESS® mini shunt surgery or trabeculectomy as a primary glaucoma surgery between March 2013 and October 2015 were included in the study, and their medical charts were retrospectively reviewed. The Ex-PRESS® and trabeculectomy groups included 14 eyes and 30 eyes, respectively. Surgical failure was defined by an intraocular pressure (IOP) of ≥21 mmHg (condition A) or ≥ 18 mmHg (condition B); Kaplan-Meier survival analyses and the multivariable Cox proportional hazards model were used to assess efficacies.
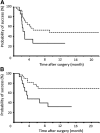
Results: Kaplan-Meier survival analyses indicated that the probabilities of success at 1 year for the Ex-PRESS® group were 25.7 and 31.8% based on complete and qualified success under condition A, respectively. The corresponding values for the trabeculectomy group were 47.8 and 69.3%, and there was a significant difference in qualified success with condition A (Fig. 1; P = 0.018), while there were no significant differences in the other criteria. Ex-PRESS® mini shunt surgery and higher intraocular pressure were independent prognostic factors using Cox proportional hazards model analyses in qualified success as in condition A (P = 0.012 and 0.0495, respectively). The occurrences of postsurgical hyphema and bleb leaks were significantly higher in the trabeculectomy group (P = 0.005 and 0.008, respectively).
Conclusion: During a 1 year follow-up, Ex-PRESS® mini shunt surgery was a less effective, but safer treatment for NVG compared with trabeculectomy.
Keywords: Efficacy; Ex-press mini shunt; Neovascular glaucoma; Safety; Trabeculectomy.
Conflict of interest statement
Ethics approval and consent to participate
All procedures conformed to the Declaration of Helsinki. This study was approved by the Institutional Review Board of Kumamoto University. Written informed consent was not obtained due to the retrospective manner of the study. Alternatively, this study was carried out by the opt-out method of our department website.
Consent for publication
Not Applicable.
Competing interests
Dr. Takahashi has received research funding from Novartis Pharma and Bayer Yakuhin. Dr. Tanihara has received consulting fees from Kowa, and MSD, board membership fees from Senju Pharmaceutical, Santen Pharmaceutical, Alcon Japan, and Pfizer Japan, and research grant from Kowa, Senju Pharmaceutical, Santen Pharmaceutical, Alcon Japan, Pfizer Japan, and Otsuka Pharmaceutical. Dr. Inoue has received research grant from Novartis Pharma. Other authors have no financial interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

