Angioedema due to acquired C1-inhibitor deficiency: spectrum and treatment with C1-inhibitor concentrate
- PMID: 30866985
- PMCID: PMC6417199
- DOI: 10.1186/s13023-019-1043-3
Angioedema due to acquired C1-inhibitor deficiency: spectrum and treatment with C1-inhibitor concentrate
Abstract
Background: Acquired angioedema due to C1-inhibitor (C1-INH) deficiency (AAE-C1-INH) is a serious condition that may result in life-threatening asphyxiation due to laryngeal edema. It is associated with malignant B-cell lymphoma and other disorders. The purpose of this study was to describe the characteristics and associated disorders of patients with AAE-C1-INH and assess the efficacy of plasma-derived C1-INH concentrate (pdC1-INH) in the treatment of AAE-C1-INH. Forty-four patients with AAE-C1-INH from the Angioedema Outpatient Service of Mainz were assessed for associated disorders. In 32 of these patients, the duration of swelling attacks was measured before and after treatment with pdC1-INH (Berinert® (CSL Behring, Marburg, Germany)). The time between injection and complete resolution of symptoms and treatment effectiveness was provided by the patients.
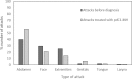
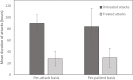
Results: The following underlying disorders were present: monoclonal gammopathy of undetermined significance (47.7%), non-Hodgkin lymphoma (27.3%), anti-C1-INH autoantibodies alone (11.4%), and other conditions (4.5%). In 9.1% patients, no associated disorder could be found. AAE-C1-INH led to the detection of lymphoma in 75% of patients with the malignancy. Treatment with pdC1-INH shortened attacks by an average (SD) 54.4 (± 32.8) hours (P < 0.0001). The earlier the attack was treated, the shorter the time between injection and resolution of symptoms (P = 0.0149). A total of 3553 (97.7%) of the 3636 attacks were effectively treated with pdC1-INH as assessed by the patient. The mean (SD) dose per-attack was 787 (± 442) U. pdC1-INH was effective in 1246 (93.8%) of 1329 attacks in 8 patients with anti-C1-INH autoantibodies and in 344 (99.4%) of 346 attacks in 6 patients without autoantibodies. The average (SD) dose per effectively treated attack was 1238.4 (± 578.2) U in patients with anti-C1-INH autoantibodies and 510.2 (± 69.1) U in patients without autoantibodies.
Conclusions: pdC1-INH is highly effective in treating AAE-C1-INH patients and is also effective in the vast majority of attacks in patients with anti-C1-INH autoantibodies. It is fast-acting and reduces attack duration.
Keywords: Acquired angioedema; C1-inhibitor concentrate, C1-inhibitor antibodies; C1-inhibitor deficiency; Non-Hodgkin lymphoma.
Conflict of interest statement
Ethics approval and consent to participate
The study was approved by the Ethics Committee of the Landesärztekammer Rheinland-Pfalz, 837.413.13 (9098-F). All patients gave their informed consent to participate in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Dobson G, Edgar D, Trinder J. Angioedema of the tongue due to acquired C1 esterase inhibitor deficiency. Anaesth Intensive Care. 2003;31:99–102. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

