Human ADSC xenograft through IL-6 secretion activates M2 macrophages responsible for the repair of damaged muscle tissue
- PMID: 30867059
- PMCID: PMC6417195
- DOI: 10.1186/s13287-019-1188-y
Human ADSC xenograft through IL-6 secretion activates M2 macrophages responsible for the repair of damaged muscle tissue
Abstract
Background: Adipose-derived mesenchymal stromal cells (ADSCs) are multipotent stromal cells. The cells secrete a number of cytokines and growth factors and show immunoregulatory and proangiogenic properties. Their properties may be used to repair damaged tissues. The aim of our work is to explain the muscle damage repair mechanism with the utilization of the human adipose-derived mesenchymal stromal cells (hADSCs).
Methods: For the hADSCs isolation, we used the subcutaneous adipose tissue collected during the surgery. The murine hind limb ischemia was used as a model. The unilateral femoral artery ligation was performed on 10-12-week-old male C57BL/6NCrl and NOD SCID mice. The mice received PBS- (controls) or 1 × 106 hADSCs. One, 3, 7, 14 and 21 days after the surgery, we collected the gastrocnemius muscles for the immunohistochemical analysis. The results were analyzed with relevant tests using the Statistica software.
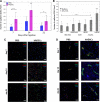
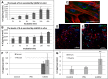
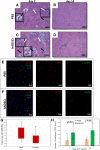
Results: The retention time of hADSCs in the limb lasted about 14 days. In the mice receiving hADSCs, the improvement in the functionality of the damaged limb occurred faster than in the control mice. More new blood vessels were formed in the limbs of the mice receiving hADSCs than in limbs of the control mice. hADSCs also increased the infiltration of the macrophages with the M2 phenotype (7-AAD-/CD45+/F4/80+/CD206+) into the ischemic limbs. hADSCs introduced into the limb of mice secreted interleukin-6. This cytokine stimulates the emergence of the proangiogenic M2 macrophages, involved, among others, in the repair of a damaged tissue. Both macrophage depletion and IL-6 blockage suppressed the therapeutic effect of hADSCs. In the mice treated with hADSCs and liposomes with clodronate (macrophages depletion), the number of capillaries formed was lower than in the mice treated with hADSCs alone. Administration of hADSCs to the mice that received siltuximab (human IL-6 blocker) did not cause an influx of the M2 macrophages, and the number of capillaries formed was at the level of the control group, as in contrast to the mice that received only the hADSCs.
Conclusions: The proposed mechanism for the repair of the damaged muscle using hADSCs is based on the activity of IL-6. In our opinion, the cytokine, secreted by the hADSCs, stimulates the M2 macrophages responsible for repairing damaged muscle and forming new blood vessels.
Keywords: Blood vessels; IL-6; M2 macrophages; Repairing damaged muscle; Xenograft; hADSCs.
Conflict of interest statement
Ethics approval and consent to participate
The experiments with animals were performed in accordance with the Declaration of Helsinki and with the approval of the Local Ethics Committee for Animal Experiments in Katowice (Permit Number: KB430–17/14).
Researches using human adipose tissue has been accepted by the Committee on the Bioethics Maria Sklodowska-Curie Institute - Oncology Center, Gliwice Branch (Permit Number: KB / 430–35 / 14).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures






References
-
- Henning RJ. Therapeutic angiogenesis: angiogenic growth factors for ischemic heart disease. Futur Cardiol. 2016;12(5):585–599. - PubMed
-
- Moon MH, Kim SY, Kim YJ, Kim SJ, Lee JB, Bae YC, Sung SM, Jung JS. Human adipose tissue-derived mesenchymal stem cells improve postnatal neovascularization in a mouse model of hindlimb ischemia. Cell Physiol Biochem. 2006;17(5–6):279–290. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

